
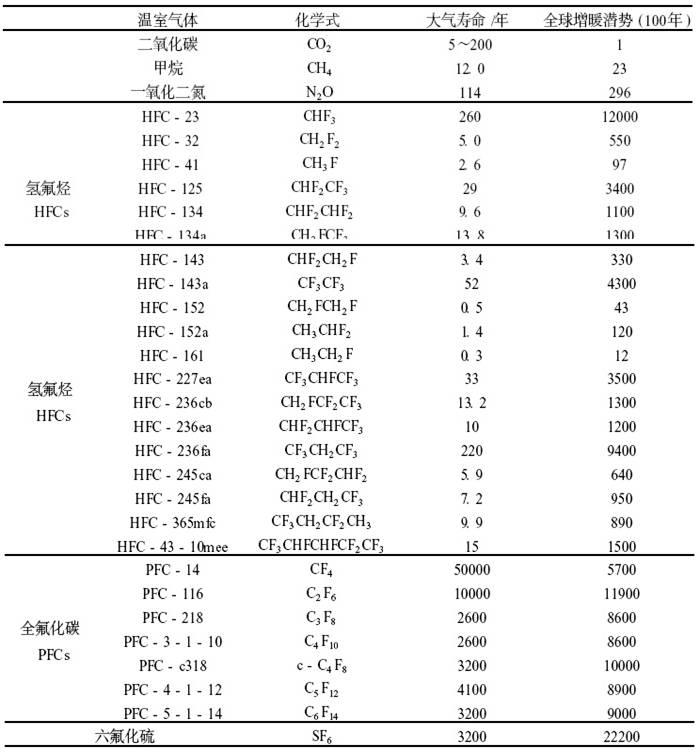
《京都议定书》要减排哪些温室气体
|
 |
悄然兴起的亚临界萃取中的液化石油气、
丙烷、二甲醚、四氟乙烷、六氟化硫萃取剂令业内人士担忧
http://www.sfst.net.cn 编辑部稿
在防火防爆类的工厂设计规范中明文规定,凡是闪点<28℃的易燃液体,凡是爆炸下限<10%的可燃性气体均应列为甲级(最高级)防火防爆等级,而目前在国內悄然兴起的亚临界萃取中,个別企业却选用闪点及爆炸下限均远远低于这两个指标的
LPG(液化石油气)、DME(二甲醚)、丙烷、R600a(高纯度异丁烷)等易燃易爆危险品,用作亚临界萃取的主溶媒,其用于固体物料的间隙式萃取,由于要频繁的装卸物料需不断的打开
、关闭萃取槽顶盖,易燃易爆气体的洩漏在所难免,同时在频繁打开 、关闭萃取槽顶盖的过程中,产生静电火花的概率也较高,在规模化的工业生产中使用这些气体,危险性是显而易见的。
在连续密闭的大规模化的生产
LPG(液化石油气)、DME(二甲醚)、丙烷、R600a(高纯度异丁烷)等
的易燃易爆气体时,相对而言由于是在连续全密闭的条件下进行操作则较为安全。
而R134a(1,1,1,2-四氟乙烷)
的亚临界萃取,由于R134a是温室气体,它能阻挡红外辐射能返回天空,对全球气候变暖直接影响较大,其全球温室效应潜能值GWP高达1300。因此早在1997年12月11日联合国气候变化框架公约缔约国第三次会议通过的《京都议定书》中,R134a是明确规定要控制与削减排放量的六
类温室气体中的一种,其排放(散发)、使用、生产也将受到严格的国际管制。再者工业级R134a的单价高于食品级二氧化碳50倍以上,作为萃取剂使用,生产成本偏高,这也是工业化生产中企业难以接受的。
更有甚者,全氟化碳PFC(如CF4和C2F6)和六氟化硫SF6也是人为产生的气体,它们大气寿命非常长,对红外辐射有强吸收。所以这些化合物虽然排放量较小,但对未来气候的影响很大。CF4在大气中可以至少存留50000年。它有自然源,但目前的人为排放是自然源的1000多倍,并且是观测到浓度增加的主要原因。一个SF6分子的温室效应是一个CO2分子的22200倍。虽然它目前的大气浓度较低(4.2×10-12),但它的增长速率明显(0.24×10-12/年)。观测到的SF6增长率与根据销售和储存数据而计算所得的排放十分一致。
[200820053135.4]超声强化超临界或亚临界流体萃取装置
[200620009043.7]亚临界汽水两相流量计
[200520021800.8]亚临界空冷汽轮机
[200710032663.1]天然产物有效成分的超声强化亚临界水萃取方法及装置
[200810088744.8]多功能亚临界压力蒸气缸
[200610104744.3]亚临界流体萃取溶剂及萃取方法/其主要特点是以液态六氟化硫为萃取溶剂/2008.04.16公开
[200610010985.1]玫瑰精油真空水散冲蒸亚临界萃取技术/2008.01.02公开
[200610081101.1]亚临界二甲醚流体提取天然除虫菊素的方法/2007.11.28公开
[200620009043.7]
亚临界汽水两相流量计/2007.10.03公开
[200610114603.X]超或亚临界下有机胺和环氧丙烷催化制备生物柴油的方法/2007.05.30公开
[200580004805.2]亚临界水分解处理物的生产方法及亚临界水分解处理物生产装置/2007.02.21公开
[200610010882.5]一种亚临界—超临界流体转化制备生物柴油的方法/2006.10.11公开
[200510061998.7]亚临界水提取中药丹参中脂溶性成分的方法/2006.08.23公开
[200510022594.7]亚临界复合热载体发生器的高温高压燃烧系统/2006.07.19公开
[200510012660.2]亚临界甲醇相固体酸碱催化油脂酯交换制生物柴油的方法/2006.01.11公开
[200310109657.3]一种低阶煤在亚临界或超临界水中连续转化的方法/2004.11.10公开
[200610070495.0]一种低压超临界萃取工艺及其装置*/本专利以1,1,1,2-四氟乙烷(R134a)作为亚临界流体萃取剂,萃取温度30~60℃萃取压力0.8~8NPa[编者按R134a:Tc:101℃;Pc:0.4MPa;其全球温室效应潜能值GWP高达1300,一些国家已禁止使用]
[200610048835.X]一种提取油脂的方法*/本专利以DME作为亚临界流体萃取剂,萃取温度30~90℃萃取压力0.5~3MPa[编者按DME:Tc:127.228℃;Pc:5.3558MPa;闪点
-41℃存在安全隐患等诸多问题]
[ 200510048681 ]-青蒿素提取的方法*
/本专利以DME作为亚临界流体萃取剂,提取温度20~60℃提取压力0.5~3MPa[编者按DME:Tc:127.228℃;Pc:5.3558MPa;闪点
-41℃存在安全隐患等诸多问题]
[ 200510048619 ]-亚临界流体处理烟草的方法*
/本专利以DME作为亚临界流体萃取剂,萃取温度20~90℃萃取压力0.5~3MPa[编者按DME:Tc:127.228℃;Pc:5.3558MPa;闪点
-41℃存在安全隐患等诸多问题]
[200410022307.8]亚临界DME精制废弃烟草初提物的方法/
2005.10.19公开/本专利以DME作为亚临界流体萃取剂,萃取温度10~60℃萃取压力0.1~1.5MPa[编者按DME:Tc:127.228℃;Pc:5.3558MPa;闪点
-41℃存在安全隐患等诸多问题,而在萃取温度10~60℃萃取压力0.1~1.5MPa的气态DME状况条件下怎能具有萃取功能?而气态DME又怎么能称之为亚临界DME?]
[200410022306.3]亚临界DME萃取废弃烟草的方法/
2005.10.19公开/本专利以DME作为亚临界流体萃取剂,萃取温度30~90℃萃取压力0.5~3MPa[编者按DME:Tc:127.228℃;Pc:5.3558MPa;闪点
-41℃存在安全隐患等诸多问题]
[ 02134162 ]-除虫菊酯精制方法*
[ 02133470 ]-印楝油提取工艺
*
[ 01108701 ]-亚临界液化石油气萃取除虫菊酯的方法*
[200810072912
]-一种生产异构化啤酒花浸膏的方法
[ 200810040260 ]-一种超高参数湿冷汽轮机
[ 200810088744 ]-多功能亚临界压力蒸气缸
[ 200810015683 ]-一种合成YAG单晶纳米粉末的方法
[ 200810085141 ]-多功能高压蒸汽仓
[ 200810019214 ]-一种酚类化合物的同步净化提取方法
[ 200720099281 ]-自然工质制冷热泵装置
[ 200710032663 ]-天然产物有效成分的超声强化亚临界水萃取方法及装置
[ 200710194357 ]-多功能中压蒸气仓
[ 200710194355 ]-多功能高压蒸气储存仓
[ 200720117563 ]-一种大型全转速汽轮机中压缸叶片使用的纵树型P叶根
[ 200720117560 ]-一种大型全转速汽轮机中压缸叶片使用的纵树型P叶根
[ 200720117568 ]-一种大型全转速汽轮机中压缸叶片使用的纵树型P叶根
[ 200710305191 ]-具有热控制安全功能的制冷或热泵回路用恒温膨胀阀
[ 200710164338 ]-亚临界压力注汽锅炉
[ 200720175748 ]-亚临界压力注汽锅炉
[ 200710113212 ]-一种耐腐蚀防堵塞的超临界水氧化反应器
[ 200710113809 ]-一种制备富含多不饱和脂肪酸的甘油酯的工艺
[ 200710049980 ]-汽轮机末级长叶片用钢材料及其热处理工艺
[ 200710066167 ]-高压流体转化技术制备生物燃料的工艺
[ 200720038457 ]-一种连通器式汽包水位取样测量装置
[ 200710025027 ]-连通器式汽包水位取样测量装置及其几何尺寸的确定方法
[ 200710049323 ]-辣椒、薯类中有效成份的提取方法
[ 200710041319 ]-热塑性淀粉及其制备方法
[ 200710098995 ]-负载型单金属加氢催化剂的水热沉积制备方法
[ 200710048828 ]-一种超超临界强化流体传热方法及传热介质
[ 200710048834 ]-一种超临界热波传热元件及其工作介质灌装密闭方法
[ 200710109730 ]-调色剂及制备方法、显影剂、成像方法和装置及处理盒
[ 200610171462 ]-反应生成物的制造方法
[ 200610114603 ]-超或亚临界下有机胺和环氧丙烷催化制备生物柴油的方法
[ 200620125242 ]-汽包水位单筒式高精度电极测量筒
[ 200620009043 ]-亚临界汽水两相流量计
[200610104744.3]亚临界流体萃取溶剂及萃取方法/其主要特点是以液态六氟化硫为萃取溶剂/2008.04.16公开
[ 200710025027 ]-连通器式汽包水位取样测量装置及其几何尺寸的确定方法
[ 200710049323 ]-辣椒、薯类中有效成份的提取方法
[ 200710109730 ]-调色剂及制备方法、显影剂、成像方法和装置及处理盒
[ 200610171462 ]-反应生成物的制造方法
[
200610010985 ]-玫瑰精油真空水散冲蒸亚临界萃取技术
[ 200610114603 ]-超或亚临界下有机胺和环氧丙烷催化制备生物柴油的方法
[
200610088092 ]-一种萃取新鲜柚子花芳香性物质的方法
[
200610035905 ]-高含量共轭不饱和三烯酸石榴籽油的制备方法
[
200610010882 ]-一种亚临界—超临界流体转化制备生物柴油的方法
[
200610010808 ]-一种便捷有效的莽草酸生产方法
[
200510022594 ]-亚临界复合热载体发生器的高温高压燃烧系统
[
200510048737 ]-天然除虫菊素水乳剂及其制备方法
[
200510061998 ]-亚临界水提取中药丹参中脂溶性成分的方法
[
200510048681 ]-青蒿素提取的方法
[
200520022024 ]-三缸超临界空冷汽轮机
[
200510048619 ]-亚临界流体处理烟草的方法
[
200510118684 ]-确定半导体制造过程的独立式结构的线宽的系统和方法
[
200520021800 ]-亚临界空冷汽轮机
[
200520021745 ]-一种大型全转速汽轮机次末级叶片
[
200510094726 ]-汽包水位大量限全工况高精度电极传感器系统
[
200510021382 ]-汽轮机末级动叶片
[
200510028318 ]-亚临界汽轮机螺栓钢细晶粒处理工艺方法
[
200510084304 ]-清洗方法及用于实施该方法的清洗装置
[
200510012660 ]-亚临界甲醇相固体酸碱催化油脂酯交换制生物柴油的方法
[
200580018196 ]-烟提取物的制备
[
200580017005 ]-二氧化碳的分解方法和碳颗粒结构体的形成方法
[
200520018534 ]-空气灭菌除臭器
[
200580015823 ]-谐振功率变换器的备用运行
[
200580009279 ]-塑料的分解方法
[
200510018433 ]-一种用于果蔬中农药残留分析的样品预处理方法和装置
[
200510052653 ]-药剂含浸方法
[
200580004805 ]-亚临界水分解处理物的生产方法及亚临界水分解处理物生产装置
[
200580004599 ]-CO*存在下的电镀
[
200580004733 ]-聚碳酸酯的分解方法
[
200510011254 ]-用于跨临界CO*制冷循环的微通道板翅式内部换热器
[
200480034060 ]-聚合物及聚合物的制造方法
[
200410095437 ]-使用致密加工流体和超声波能处理半导体元件的方法
[
200480038727 ]-活性聚合物挤出物的制备
[
200410056082 ]-脂肪酸脂的制造方法
[
200410055063 ]-含有有机物的废水的净化处理方法
[
200410022306 ]-亚临界DME萃取废弃烟草的方法
[
200410022307 ]-亚临界DME精制废弃烟草初提物的方法
[
200410035253 ]-纳米碳材料的制备方法
[
200510127455 ]-生产乙酸纤维素的方法及其植物组分提取装置
[
200410008917 ]-制造纤维素模塑件的法,植物组分提取装置,和生产乙酸纤维素的方法
[
200510127454 ]-植物组分提取装置
[
200410016559 ]-二氧化碳电冰箱
[
200410055087 ]-制造纳米碳材料的方法
[ 03801005 ]-聚合物的制造方法
[ 200380110258 ]-树脂微粒的制造方法和树脂微粒
[
200310109657 ]-一种低阶煤在亚临界或超临界水中连续转化的方法
[
200380102845 ]-聚合物的分解方法
[
200380101897 ]-甲烷气的制造方法
[
200310107844 ]-超临界空冷汽轮机
[
200310107845 ]-超超临界空冷汽轮机
[ 03817167
]-烟草中成分的减少
[ 03820704
]-颜料纳米粒子的新型制造方法
[ 02134162
]-除虫菊酯精制方法
[ 02158446 ]-富勒烯的制备方法
[ 02154271
]-纳米碳材料的制备方法
[ 02126631
]-亚临界转速球磨机
[ 02274303
]-生产脂质体的设备
[ 02132650
]-脂质体的生产工艺及设备
[ 02133470
]-印楝油提取工艺
[ 02801115
]-用喷射临界状态的水来运行内燃机的方法
[ 02806085
]-利用超临界流体或亚临界流体的有机物质等的反应装置
[ 01815350
]-水热氧化处理废物的方法
[ 01108701
]-亚临界液化石油气萃取除虫菊酯的方法
[ 01206543
]-大型汽轮机新型末级叶片
[ 01122803
]-硬质聚氨酯泡沫塑料的原料制造方法,冷藏库的制造方法和冷藏库
[ 01113903
]-在亚临界状态下用CO*从银杏叶中萃取黄酮和萜类的方法
[ 00268712
]-采用波纹管的超临界水氧化法污水处理反应器
[ 00221057
]-汽包水位高精度取样电极传感器
[ 00100676
]-异物除去法及膜形成方法
[ 99123246
]-与布线亚临界接触的自对准工艺
[ 98117610
]-废弃物处理方法和废弃物处理装置
[
200510075433 ]-废弃物处理方法和废弃物处理装置
[ 98806386
]-在超临界流体中制得的生物功能聚合物
[ 98101649
]-缓和热转化--溶剂脱沥青组合工艺
[ 98804492
]-超临界反应装置及其方法
[ 98106777
]-一种费一托合成催化剂的原位再生法
[ 97198781
]-一种粉末状制剂的制备工艺
[ 97116908
]-多元高铬铸钢轧钢机组合式导板及其制造方法
[ 97225687
]-具有亚临界叶型的离心式前、后向叶轮
[ 97225208
]-无盲区云母双色水位计
[ 97217502
]-板式云母双色水位计
[ 96198262
]-制备丙烯均聚物或共聚物的方法
[ 96102285
]-蒸汽发生装置
[ 96107763
]-燃烧室
[ 96195038
]-环滚研磨机
[ 96194418
]-计量喷雾阀
[ 96192192
]-改性气凝胶的制备方法及其应用
[ 95242232
]-涡流式超临界、亚临界气体萃取装置
[ 94238937 ]-适用于任何工况的差压式水位测量元件
[ 94102373
]-一种γ-Fe2O3磁粉的制备方法
[ 94110049
]-耐低温可焊接细晶粒厚度方向钢板
[ 90106041
]-板式云母双色水位计
[ 90218673
]-具有亚临界叶型的离心式后向叶轮
[ 90100948
]-溶剂萃取方法
[ 88103142
]-双色照明双色液位计的双色照明方法和这种双色液位计
[ 87209305
]-扣合内通道式窥视镜体液位计
[ 86202977
]-一种反射.透射式双色液位计
Subcritical
Subcritical water chromatography: A green approach to
high-temperature liquid chromatography.
At
temperatures and pressures lower than 374 degrees C and
218 atm, subcritical water has widely tunable properties
such as dielectric constant, surface tension, viscosity,
and dissociation constant achieved by simply adjusting
the temperature with a moderate pressure to keep water
in the liquid state. At elevated temperatures, water
acts like a weak polar organic solvent. Thus,
subcritical water has been used as a green eluent to
replace hazardous solvents commonly used as organic
modifiers in RPLC. Subcritical water chromatography (SBWC)
is capable of separating polar, moderately polar, and
even some nonpolar analytes. Most of these low molecular
weight solutes are stable at elevated temperatures
during a chromatographic run. Some new packing materials
are also quite stable and robust at mild temperatures
ranging from 80 to 150 degrees C. Advantages of SBWC
include the elimination of hazardous organic solvents
used in traditional RPLC, rapid analysis time, improved
selectivity, temperature-dependent separation
efficiency, temperature-programmed elution, and
compatibility with both gas- and liquid-phase detectors.
In this paper, the technical aspects as well as the
applications of SBWC are reviewed. Topics addressed in
this review include the unique characteristics of
subcritical water, analytes separated by SBWC, packing
materials tested for SBWC, the application of GC and LC
detection techniques in SBWC, SBWC instrumentation
development, temperature effects on SBWC separation, and
models developed for separation in SBWC.
Rapid column heating method for subcritical water
chromatography.
A novel
resistive heating method is presented for subcritical
water chromatography (SWC) that provides higher column
heating rates than those conventionally obtained from
temperature-programmed gas chromatography (GC)
convection ovens. Since the polarity of water reduces
dramatically with increasing temperature, SWC employs
column heating to achieve gradient elution. As such, the
rate at which the mobile phase is heated directly
impacts the magnitude of such gradients applied in SWC.
Data from the current study demonstrate that the maximum
column heating rate attainable in a typical SWC
apparatus (i.e. using a GC convection oven) is around 10
degrees C/min, even at instrument oven settings of over
three times this value. Conversely, by wrapping the
separation column with ceramic insulation and a
resistively heated wire, the column heating rates are
increased five-fold. As a result, elution times can be
greatly decreased in SWC employing gradients.
Separations of standard alcohol test mixtures
demonstrate that the retention time of the latest
eluting component decreases by 35 to 50% using the
prototype method. Additionally, solute retention times
in this mode deviate by less than 1% RSD over several
trials, which compares very well to those obtained using
a conventional GC convection oven. Results suggest that
the developed method can be a useful alternative heating
technique in SWC.
Hydrolysis kinetics of trisaccharides consisting of
glucose, galactose, and fructose residues in subcritical
water.
The
hydrolysis kinetics of trisaccharides consisting of
glucose, galactose, and fructose residues with different
glycosidic bonds, 1-kestose, d-melezitose, d-raffinose,
and lactosucrose, in subcritical water were conducted
over the temperature range of 150-230 degrees C and at a
constant pressure of 10 MPa. The hydrolysis of
trisaccharides in subcritical water proceeded
consecutively, i.e., one cleavage of the two bonds
antedated the other. The preceding cleavage was not
expressed by the first-order kinetics, but by the
kinetics considering the concentration of the acidic
compounds, which were produced by the degradation of the
constituent monosaccharides. The hydrolysis of the
constituent disaccharides, except sucrose composed of
the alpha-Glc-(1-->2)-beta-Fru bond, obeyed first-order
kinetics. All of the rate constants of the hydrolytic
kinetics were determined, and the values were found to
depend on the type of bond.
Subcritical water extraction of nutraceuticals with
antioxidant activity from oregano. Chemical and
functional characterization.
In the
present work, oregano leaves (Origanum vulgare L.) are
explored as natural source of nutraceuticals with
antioxidant activity. To do this, subcritical water
extraction (SWE), a new environmentally friendly
technique, is employed as extraction procedure and HPLC
coupled to DAD is used for the chemical characterization
of the extracts. Moreover, the radical scavenging
1,1-diphenyl-2-picrylhydrazyl (DPPH) method and the
determination of the total phenolic content (measured
with the Folin test) are applied to evaluate the
antioxidant activity of the extracts. The extraction of
antioxidants from oregano leaves by SWE is studied
considering different temperatures (25, 50, 100, 150 and
200 degrees C) to investigate the selectivity of the
process. The highest antioxidant activity is observed
for the extract obtained at the highest temperature, 200
degrees C (EC(50) equal to 10 microg/ml). Moreover, the
extraction yield was also the highest (54% dry weight)
at these extraction conditions. The total phenolic
content showed no differences among the different
extracts, concluding that the amount of phenolic
compounds extracted was similar but the type and
structure of the phenolics was different, providing in
this way different antioxidant activity. Some compounds
could be tentatively identified, proposing some probable
chemical structures for some of them, such as flavanones,
dihydroflavonols, favonols and flavones.
Development of pressurized subcritical water extraction
combined with stir bar sorptive extraction for the
analysis of organochlorine pesticides and chlorobenzenes
in soils.
An
analytical method for the determination of several
organochlorine pesticides (OCPs) like
hexachlorocyclohexanes (HCHs), cyclodiene derivates (dieldrin,
aldrin, endrin, heptachlor, heptachlor epoxide, endrin
aldehyde, endosulfan and ensodulfan sulphate) and DDX
compounds (p,p'-DDE, p,p'-DDD and p,p'-DDT) as well as
chlorobenzenes in soils has been developed. The
procedure is based on pressurized subcritical water
extraction (PSWE) followed by stir bar sorptive
extraction (SBSE) and subsequent thermodesorption-gas
chromatography/mass spectrometry analysis. Significant
PSWE and SBSE parameters were optimized using spiked
soil and water samples. For the PSWE of the
organochlorine compounds, water modified with
acetonitrile as the extraction solvent, at an extraction
temperature of 120 degrees C, and three cycles of 10 min
extraction proved to be optimal. Under optimized
conditions, the figures of merit, such as precision,
accuracy and detection limits were evaluated. The
detection limits obtained for soil samples were in the
range 0.002-4.7 ng/g. Recoveries between 4.1 and 85.2%
were achieved from samples spiked at a concentration
level of 25-155 ng/g. The main advantages of this method
are the avoidance of clean-up and concentration
procedures as well as the significant reduction of the
required volume of organic solvents. The described
method was applied to the determination of the
pollutants in soil samples collected from a polluted
area, the Bitterfeld region (Germany). The results
obtained by PSWE-SBSE were in a good agreement with
those obtained by a reference method, a conventional
pressurized liquid extraction (PLE).
Efficient decomposition of environmentally persistent
perfluorooctanesulfonate and related fluorochemicals
using zerovalent iron in subcritical water.
Decomposition of perfluorooctanesulfonate (PFOS) and
related chemicals in subcritical water was investigated.
Although PFOS demonstrated little reactivity in pure
subcritical water, addition of zerovalent metals to the
reaction system enhanced the PFOS decomposition to form
F-ions, with an increasing order of activity of no metal
approximately equal Al < Cu < Zn << Fe. Use of iron led
to the most efficient PFOS decomposition: When iron
powder was added to an aqueous solution of PFOS (93-372
microM) and the mixture was heated at 350 degrees C for
6 h, PFOS concentration in the reaction solution fell
below 2.2 microM (detection limit of HPLC with
conductometric detection), with formation of F-ions with
yields [i.e., (moles of F- formed)/(moles of fluorine
content in initial PFOS) x 100] of 46.2-51.4% and
without any formation of perfluorocarboxylic acids. A
small amount of CHF3 was detected in the gas phase with
a yield [i.e., (moles of CHF3)/(moles of carbon content
in initial PFOS) x 100] of 0.7%, after the reaction of
PFOS (372 microM) with iron at 350 degree C for 6 h.
Spectroscopic measurements indicated that PFOS in water
markedly adsorbed on the iron surface even at room
temperature, and the adsorbed fluorinated species on the
iron surface decomposed with rising temperature, with
prominent release of F- ions to the solution phase above
250 degrees C. This method was also effective in
decomposing other perfluoroalkylsulfonates bearing
shorter chain (C2-C6) perfluoroalkyl groups and was
successfully applied to the decomposition of PFOS
contained in an antireflective coating agent used in
semiconductor manufacturing.
Resource recovery from excess sludge by subcritical
water combined with magnesium ammonium phosphate
process.
The amount
of excess sludge produced in municipal wastewater
treatment plants in Japan is increasing every year as
the urban population increases. Phosphorus in excess
sludge could be a potential phosphorus resource since at
present, phosphate rock is being exhausted all over the
world. Every year, Japan imports large quantities of
phosphorus from abroad but much is discharged as excess
sludge. Therefore, the solubilization process, one
method of recovering phosphorus from sludge, could be a
promising solution. In this study, a subcritical water
process, a new technology that solubilizes sludge under
subcritical conditions, was applied before the
phosphorus in sludge was recovered with the magnesium
ammonium phosphate (MAP) process. As a result, the
solubilization rate of excess sludge achieved
approximately 80% and about 94-97% of the phosphorus
could be recovered.
Rapid determination of inorganic elements in airborne
particulate matter by using acidified subcritical-water
extraction and inductively-coupled
plasma-optical-emission spectrometry.
A rapid and
simple method has been developed for determination of
inorganic elements in airborne particulate matter (PM10)
by using acidified subcritical water and ICP-OES.
Elements such as Al, As, B, Ba, Cd, Cu, Fe, Mn, Pb, Se,
and Zn were rapidly and efficiently extracted from PM10
samples with a solution of 0.1 mol L(-1) HNO(3) under
subcritical conditions. The method requires
approximately 5% of the amount of acid used in the
standard microwave extraction procedure. The material
selected for the subcritical extraction manifold was
poly ether ether ketone (PEEK), to avoid sample
contamination with elements present in previously
reported stainless-steel manifolds. The extraction
temperature, time of static and dynamic extraction, and
flow rate of acidified water were studied keeping the
pressure controlled at about 1,500 psig. The efficiency
of extraction of most of the analytes increased with
temperature, tending to quantitative extraction at
temperatures near 150 degrees C. After the extraction
process the analytes were determined directly in the
extract by ICP-OES. When the method was compared with
the USEPA counterpart, the results indicate that under
optimized conditions (static extraction time: 15 min,
dynamic extraction time: 30 min, flow rate: 2 mL
min(-1)) the analytes were extracted with recoveries
between 73 and 158%. Alternatively, by using an
extraction time of 15 min, the method could be used to
screen for all the elements, with recoveries over 50%.
The developed method was applied to the determination of
inorganic elements in airborne particulate matter in the
atmosphere of Santiago, Chile.
Decomposition kinetics of maltose in subcritical water.
The
decomposition process of maltose in subcritical water
was studied using a tubular reactor in the temperature
range of 180 to 260 degrees C and at 10 MPa. The
formation of glucose and 5-hydroxymethyl-2-furaldehyde
during the maltose decomposition was also observed. The
decomposition rate of maltose was faster at higher
temperatures. The rate was approximated by first-order
kinetics during the early stage of the decomposition,
but was accelerated and deviated from these kinetics at
the later stage. The effluent pH decreased as the
residence time in the reactor increased and the decrease
of pH affected the maltose decomposition rate and
glucose formation. Low pH of a feed solution accelerated
maltose decomposition. A good correlation was obtained
between the pH of the effluent and the rate constant of
the first-order kinetics.
Hydrolysis of ginger bagasse starch in subcritical water
and carbon dioxide.
Ginger
bagasse from supercritical extraction was hydrolyzed
using subcritical water and CO(2) to produce reducing
sugars and other low molecular mass substances. Response
surface methodology was used to find the best hydrolysis
conditions; the degree of hydrolysis and the yield were
the two response variables selected for maximization.
The kinetic studies of the hydrolysis were performed at
150 bar and temperatures of 176, 188, and 200 degrees C.
The higher degree of hydrolysis (97.1% after 15 min of
reaction) and higher reducing sugars yield (18.1% after
11 min of reaction) were established for the higher
process temperature (200 degrees C). Different mixtures
of oligosaccharides with different molecular mass
distributions were obtained, depending on the
temperature and on the reaction time. The ginger bagasse
hydrolysis was treated as a heterogeneous reaction with
a first-order global chemical kinetic, in relation to
the starch concentration, which resulted in an
activation energy of 180.2 kJ/mol and a preexponential
factor of 5.79 x 10(17)/s.
Subcritical (hot/liquid) water dechlorination of PCBs (Aroclor
1254) with metal additives and in waste paint.
No disposal
option exists for "mixed wastes" such as paint scrapings
that are co-contaminated with polychlorinated biphenyls
(PCBs) and radioactive metals. Either removal or
destruction of the PCBs is required prior to disposal.
Comparison of subcritical water dechlorination (350
degrees C, 1 h) of Aroclor 1254 in paint scrapings (180
ppm) and of standard Aroclor 1254 showed significantly
enhanced dechlorination in the presence of paint. While
no significant degradation was observed for standard
Aroclor (no paint), the dechlorination of PCBs in paint
was 99, 99, and 80% for the hepta-, hexa-, and
pentachlorinated congeners, respectively, indicating
that metals in the paint enhanced the dechlorination
reactions. Adding metals to the standard Aroclor (no
paint) reactions enhanced PCB dechlorination in
subcritical water in descending order of activity: Pb
approximately = Cu > Al > Zn > Fe. In the presence of
both zerovalent and divalent lead and zerovalent copper
in subcritical water (350 degrees C, 1 h), 99% of the
Aroclor 1254 mixture (tetra- to heptachlorinated
biphenyls) was dechlorinated. High dechlorination (ca.
95%) was also achieved with zerovalent aluminum. In
contrast to other metals, lead retained its degradation
ability at a lower temperature of 250 degrees C after 18
h. The high degradation efficiency achieved using metal
additives in water at reasonable temperatures and
pressures demonstrates the potential for subcritical
water dechlorination of PCBs in paint scrapings and,
potentially, in other solid and liquid wastes.
Off-line coupling of subcritical water extraction with
subcritical water chromatography via a sorbent trap and
thermal desorption.
In this
study, the off-line coupling of subcritical water
extraction (SBWE) with subcritical water chromatography
(SBWC) was achieved using a sorbent trap and thermal
desorption. The sorbent trap was employed to collect the
extracted analytes during subcritical water extraction.
After the extraction, the trap was connected to the
subcritical water chromatography system, and thermal
desorption of the trapped analytes was performed before
the SBWC run. The thermally desorbed analytes were then
introduced into the subcritical water separation column
and detected by a UV detector. Anilines and phenols were
extracted from sand and analyzed using this off-line
coupling technique. Subcritical water extraction of
flavones from orange peel followed by subcritical water
chromatographic separation was also investigated. The
effects of water volume and extraction temperature on
flavone recovery were determined. Because a sorbent trap
was used to collect the extracted analytes, the
sensitivity of this technique was greatly enhanced as
compared to that of subcritical water extraction with
solvent trapping. Since no organic solvent-water
extractions were necessary prior to analysis, this
technique eliminated any use of organic solvents in both
extraction and chromatography processes.
Subcritical water extraction of antioxidant compounds
from rosemary plants.
Subcritical water extraction at several
temperatures ranging from 25 to 200 degrees C has been
studied to selectively extract antioxidant compounds
from rosemary leaves. An exhaustive characterization of
the fractions obtained using subcritical water at
different temperatures has been carried out by LC-MS,
and the antioxidant activities of the extracts have been
measured by a free radical method (DPPH). Results
indicate high selectivity of the subcritical water
toward the most active compounds of rosemary such as
carnosol, rosmanol, carnosic acid, methyl carnosate, and
some flavonoids such as cirsimaritin and genkwanin. The
antioxidant activity of the fractions obtained by
extraction at different water temperatures was very
high, with values around 11.3 microg/mL, comparable to
those achieved by SFE of rosemary leaves. A study of the
effect of the temperature on the extraction efficiency
of the most typical rosemary antioxidant compounds has
been performed.
Alkaline subcritical-water treatment and alkaline heat
treatment for the increase in biodegradability of
newsprint waste.
This work
describes two alkaline semicontinuous processes for the
conversion of refractory organic materials into
biodegradable substances. Newsprint was used as a
lignocellulosic waste. Methane conversion efficiencies
and cellulose removals were investigated for the two
following processes: alkaline subcritical-water
treatment (ASWT) coupled with methane fermentation and
alkaline heat treatment (newsprint heated with steam in
an autoclave; AHT) coupled with methane fermentation
with a neutral subcritical-water treatment (NSWT)
recycle. Results showed that for ASWT coupled with
methane fermentation higher methane conversion
efficiencies and higher cellulose removals were achieved
as HRT increased. At HRT = 20 days, average CH4
conversion efficiency and average cellulose removal
reached 26% and 44%, respectively. After a final HRT of
40 days, average CH4 conversion efficiency and average
cellulose removal reached 50% and 60%, respectively. On
the other hand, for AHT coupled with methane
fermentation, methane conversion efficiencies did not
show a greater improvement using this pretreatment
process. Average conversion reached 9% with an average
cellulose removal of 20%. In order to improve the yield
of the reactor, approximately one-third of the effluent
was recycled using NSWT (150 degrees C; neutral pH).
Methane conversion efficiency of this process increased
as more recycles were performed. For the fifth
operation, the total average methane conversion
efficiency was 44% with a total average cellulose
removal of 55%.
A novel process utilizing subcritical water and
nitrilotriacetic acid to extract hazardous elements from
MSW incinerator fly ash.
An
effective process for hazardous element extraction from
municipal solid waste incinerator fly ash was developed.
The key trait of the process was to extract most of the
hazardous elements out of the ash but leave Ca and Si
inside the residue. In the extraction process, the ash
was firstly pre-washed with water, then subjected to
subcritical water (SC water) treatment and
nitrilotriacetic acid (NTA) extraction. SEM images
indicated that SC water is strong enough to destroy the
ash particles, thus greatly improving hazardous elements
extraction efficiency in the subsequent NTA extraction
process. The extraction percentages for Cr, As, Se, Cd
and Pb under SC water+NTA treatment were around 2-6
times higher than those treated by NTA at room
conditions. The preferable SC treatment temperature was
573 K and the treatment time was 3 h. The optimum NTA
concentration, vibration time and liquid/solid ratio
were 0.8 M, 5 h and 10:1 (ml/g), respectively.
Furthermore, it was found that introduction of a
suitable amount of sulfuric acid into the extraction
solution could extract more than 90% of most of the
hazardous elements out of the ash.
Reductive dechlorination of polychlorinated dibenzo-p-dioxins
by zerovalent iron in subcritical water.
A new
method for reductive dechlorination of polychlorinated
dibenzo-p-dioxins (PCDDs) and remediation of
contaminated soils is described that uses zerovalent
iron as the dechlorination agent and subcritical water
as reaction medium and extractive solvent. It is found
that the zerovalent iron can be applied for stepwise
dechlorination of octachlorinated dibenzo-p-dioxin (OCDD)
on various matrixes in subcritical water. By using iron
powder as matrix higher chlorinated congeners were
practically completely reduced to less than
tetra-substituted homologues. A significant part of
residual OCDD, when it was spiked in to soils, and
formed less chlorinated congeners are extracted with
water in the given conditions. The solubility of OCDD
was increased by a 4-6 orders over its solubility at
ambient conditions. The new method of contentious-flow
extraction is described.
Subcritical water extraction and determination of
nifedipine in pharmaceutical formulations.
A rapid and
simple continuous method for the extraction of
nifedipine from tablets was developed by using
pressurized hot water at 150 degrees C. This is the
first time that subcritical water was applied to the
extraction of low-polarity compounds in pharmaceutical
analysis. The method is based on the increment in
solubility of nifedipine in subcritical water.
Extraction temperature and static and dynamic extraction
time were optimized in order to reach quantitative
extraction of the drug from the tablets. After
extraction, the drug was determined by spectrophotometry
by measuring absorbance at 338 nm. Accuracy and
precision of the method were determined by analysis of
10 synthetic samples of pharmaceutical formulations
prepared with common tablet excipients. Recovery was
found to be 99.2% with a relative standard deviation of
1.9%, which indicates that the excipients of the
formulation do not interfere in the determination. The
method was applied to the determination of the drug
content uniformity in tablets.
Subcritical water extraction of essential oils from
coriander seeds (Coriandrum sativum L.)
Subcritical water extraction (SCWE),
hydrodistillation and Soxhlet extraction were compared
for the extraction of essential oil from coriander seeds
(Coriandrum sativum L.). The extraction
efficiencies of different temperatures (100, 125, 150
and 175 °C), mean particle sizes (0.25, 0.50 and 1 mm),
and water flow rates (1, 2 and 4 ml/min) were
investigated. Separation and identification of the
components were carried out by GC–FID and GC–MS. The
results showed that the optimum temperature, mean
particle size, and flow rate were 125 °C, 0.5 mm, and
2 ml/min. The SCWE was compared with both conventional
methods in terms of the efficiency and the essential oil
composition. Hydrodistillation and Soxhlet extraction
showed higher extraction efficiencies, but the SCWE
resulted to the essential oils more concentrated in
valuable oxygenated components.
Ethanol-modified subcritical water extraction combined
with solid-phase microextraction for determining
atrazine in beef kidney.
The
determination of the levels of pesticides in food
products has prompted the development of sensitive and
rapid methods of analysis that are solvent-free or
utilize solvents that are benign to the environment and
laboratory worker. In this study we have developed a
novel extraction method that utilizes ethanol-modified
subcritical water in combination with solid-phase
microextraction (SPME) for the removal of atrazine from
beef kidney. In situ sample cleanup was achieved using
the technique of matrix solid-phase dispersion. A
cross-linked polymer, XAD-7 HP, was utilized as a
dispersing material for kidney samples. Subcritical
water extractions were performed with a pressurized
solvent extraction unit at 100 degrees C and 50 atm.
Experimental parameters investigated were the volume of
solvent and amount of modifier required for the complete
extraction of atrazine and optimization of the
extraction time. It was determined that 30% ethanol in
water (v/v) is adequate for the complete extraction of
atrazine. A Carbowax-divinylbenzene SPME fiber was used
to sample the aqueous extracts. Analysis of the fiber
contents was by ion-trap GC/MS utilizing the single ion
mode. The total time of analysis for a single kidney
sample is 90 min. The average percent recoveries from
samples spiked to the concentrations of 2 and 0.2 microg/g
were 104 and 111, respectively. The average relative
standard deviations were 10 and 9, respectively. The
method limit of detection for beef kidney spiked with
atrazine was found to be 20 ng/g of sample.
Extraction of tricyclazole from soil and sediment with
subcritical water.
The use of
subcritical water to extract tricyclazole from soils and
sediments was examined. Extraction efficiency and
kinetics were determined as a function of temperature,
sample age, sample matrix, sample size, and flow rate.
Extraction temperature was the most influential
experimental factor affecting extraction efficiency and
kinetics, with increasing temperature (up to 150 degrees
C) yielding faster and higher efficiency extractions.
Higher extraction temperatures were also important for
quantitative recovery of tricyclazole from aged samples.
Extraction at 50 degrees C yielded 97% recoveries from
samples aged 1 day but only 30% recoveries for samples
aged 202 days, whereas extraction at 150 degrees C
yielded recoveries of 85-100% that were independent of
incubation time and sample matrix, with the exception of
one sediment that contained a large amount of organic
matter. Sample extracts from subcritical water
extraction were generally a pale yellow color,
contrasted with a dark brown color from organic solvent
extractions of the same matrixes. Less sample cleanup
was therefore required prior to analysis, with the total
time for the extraction and analysis of a single sample
being approximately 2 h. Subcritical water extraction is
an effective technique for the rapid and quantitative
extraction of tricyclazole from soils and sediments.
Continuous subcritical water extraction of medicinal
plant essential oil: comparison with conventional
techniques
A
subcritical extractor equipped with a three-way inlet
valve and an on/off outlet valve has been used for
performing subcritical water extractions (SWE) in a
continuous manner for the isolation of the essential oil
of fennel, a medicinal plant. The target compounds were
removed from the aqueous extract by a single extraction
with 5 ml hexane, determined by gas-chromatography-flame
ionization (GC-FID) and identified by mass spectrometry
(MS). The proposed extraction method has been compared
with both hydrodistillation and dichloromethane manual
extraction. Better results have been obtained with the
proposed method in terms of rapidity, efficiency,
cleanliness and possibility of manipulating the
composition of the extract.
Characteristics of lithium iron phosphate (LiFePO4)
particles synthesized in subcritical and supercritical
water
The effect
of temperature, pH, time, and reactant concentrations on
the size and morphology of lithium iron phosphate
(LiFePO4) particles synthesized in a batch
hydrothermal reactor was investigated in this work. It
was found that LiFePO4 could only be
synthesized at neutral or slightly basic pH in both
subcritical and supercritical water. Synthesis in
subcritical water resulted in micron-sized particles of
high crystallinity, whereas synthesis in supercritical
water produced submicron particles. A more uniform
particle size distribution was obtained at low reactant
concentrations, irrespective of the synthesis
temperature. Qualitative explanations for these
observations are provided in terms of nucleation,
growth, and agglomeration phenomena at subcritical and
supercritical conditions.
Results of Search in US Patent
Collection db for:
"Subcritical": patents.
| |
PAT. NO. |
|
Title |
| 1 |
7,387,761 |
 |
Method for
manufacturing a glass infiltrated metal oxide
infrastructure
|
| 2 |
7,386,985 |
 |
Detection of
refrigerant charge adequacy based on multiple
temperature measurements
|
| 3 |
7,385,057 |
 |
Method and
device for producing melamine in a single-phase
tubular reactor
|
| 4 |
7,384,484 |
 |
Substrate
processing method, substrate processing
apparatus and substrate processing system
|
| 5 |
7,381,439 |
 |
Method and
composition for washing poultry during
processing
|
| 6 |
7,381,320 |
 |
Heavy oil
and bitumen upgrading
|
| 7 |
7,381,278 |
 |
Using
supercritical fluids to clean lenses and monitor
defects |
| 8 |
7,374,900 |
 |
Fluorescent
substrates for detecting organophosphatase
enzyme activity
|
| 9 |
7,365,236 |
 |
Generation
of a creosote-like mixture, or recovery of
metals, or both from preserved wood by reaction
in supercritical water
|
| 10 |
7,365,234 |
 |
Tuning
product selectivity in catalytic
hydroformylation reactions with carbon dioxide
expanded liquids
|
| 11 |
7,364,839 |
 |
Method for
forming a pattern and substrate-processing
apparatus |
| 12 |
7,361,231 |
 |
System and
method for mid-pressure dense phase gas and
ultrasonic cleaning
|
| 13 |
7,360,420 |
 |
Method and
bearing for balancing rotors without journals
|
| 14 |
7,353,113 |
 |
System,
method and computer program product for aquatic
environment assessment
|
| 15 |
7,349,517 |
 |
System for
measuring burn-out of fuel elements of a
high-temperature reactor
|
| 16 |
7,347,193 |
 |
Method and
device for determining the mass flow rate
passing through the air-bleed valve of an
internal combustion engine tank
|
| 17 |
7,344,746 |
 |
Process for
the hydrogenation of hop resin acids
|
| 18 |
7,337,742 |
 |
Twin fin
fairing |
| 19 |
7,337,356 |
 |
Systematic
and random error detection and recovery within
processing stages of an integrated circuit
|
| 20 |
7,335,296 |
 |
System and
device for processing supercritical and
subcritical fluid
|
| 21 |
7,335,287 |
 |
Solid
electrolyte sensor for monitoring the
concentration of an element in a fluid
particularly molten metal
|
| 22 |
7,334,430 |
 |
Refrigerating cycle
|
| 23 |
7,331,313 |
 |
Continuous
steam generator with circulating atmospheric
fluidised-bed combustion
|
| 24 |
7,326,756 |
 |
High
temperature bulk polymerization of branched
crystalline polypropylene
|
| 25 |
7,326,337 |
 |
System for
oxidation of organic bodies present in an
aqueous effluent
|
| 26 |
7,326,001 |
 |
Wave forming
apparatus and method
|
| 27 |
7,323,096 |
 |
Method for
treating the surface of object and apparatus
thereof |
| 28 |
7,322,101 |
 |
Turbine
engine disk spacers
|
| 29 |
7,320,229 |
 |
Ejector
refrigeration cycle
|
| 30 |
7,320,091 |
 |
Error
recovery within processing stages of an
integrated circuit
|
| 31 |
7,319,125 |
 |
Supercritical polymerization process and
polymers produced therefrom
|
| 32 |
7,316,824 |
 |
Method and
composition for washing poultry during
processing
|
| 33 |
7,316,769 |
 |
Length-dependent recoil separation of long
molecules |
| 34 |
7,314,942 |
 |
Methylenelactones syntheses in supercritical
fluids |
| 35 |
7,311,003 |
 |
Method and
device for balancing journal-less rotors
|
| 36 |
7,310,755 |
 |
Data
retention latch provision within integrated
circuits |
| 37 |
7,304,309 |
 |
Radiation
detectors |
| 38 |
7,300,527 |
 |
Method for
activating surface of base material and
apparatus thereof
|
| 39 |
7,299,913 |
 |
Drive unit
for a vibrating spiral conveyor
|
| 40 |
7,294,353 |
 |
Methods and
compositions comprising ilex
|
| 41 |
7,293,403 |
 |
Combustion
chamber with internal jacket made of a ceramic
composite material and process for manufacture
|
| 42 |
7,291,890 |
 |
Gate
dielectric and method
|
| 43 |
7,291,296 |
 |
Method for
making very fine particles consisting of a
principle inserted in a host molecule
|
| 44 |
7,288,237 |
 |
Epoxidation
catalyst |
| 45 |
7,287,725 |
 |
Missile
control system and method
|
| 46 |
7,287,541 |
 |
Method,
apparatus and system for controlling fluid flow
|
| 47 |
7,287,381 |
 |
Power
recovery and energy conversion systems and
methods of using same
|
| 48 |
7,285,312 |
 |
Atomic layer
deposition for turbine components
|
| 49 |
7,282,099 |
 |
Dense phase
processing fluids for microelectronic component
manufacture
|
| 50 |
7,281,484 |
 |
Multimission
transonic hull and hydrofield
|
| 51 |
7,279,184 |
 |
Methods and
compositions comprising Ilex
|
| 52 |
7,279,127 |
 |
Continuous
steelmaking plant
|
| 53 |
7,278,264 |
 |
Process to
convert low grade heat source into power using
dense fluid expander
|
| 54 |
7,276,184 |
 |
Surfactant
assisted nanomaterial generation process
|
| 55 |
7,275,400 |
 |
Washing
apparatus |
| 56 |
7,273,826 |
 |
Epoxidation
catalyst |
| 57 |
7,270,795 |
 |
Method for
producing nano-carbon materials
|
| 58 |
7,267,727 |
 |
Processing
of semiconductor components with dense
processing fluids and ultrasonic energy
|
| 59 |
7,264,710 |
 |
Process and
apparatus for treating heavy oil with
supercritical water and power generation system
equipped with heavy oil treating apparatus
|
| 60 |
7,263,727 |
 |
Hygienic
high detergency toilet
|
| 61 |
7,262,422 |
 |
Use of
supercritical fluid to dry wafer and clean lens
in immersion lithography
|
| 62 |
7,261,529 |
 |
Apparatus
for preparing biodegradable microparticle
formulations containing pharmaceutically active
agents |
| 63 |
7,260,001 |
 |
Memory
system having fast and slow data reading
mechanisms
|
| 64 |
7,259,231 |
 |
Extraction
and fractionation of biopolymers and resins from
plant materials
|
| 65 |
7,259,136 |
 |
Compositions
and methods for treating peripheral vascular
disease |
| 66 |
7,258,791 |
 |
Method of
reducing volume of sludge
|
| 67 |
7,252,719 |
 |
High
pressure processing method
|
| 68 |
7,244,461 |
 |
Method of
preparing liquid smoke
|
| 69 |
7,243,618 |
 |
Steam
generator with hybrid circulation
|
| 70 |
7,243,501 |
 |
Expansion
device for an air-conditioning system
|
| 71 |
7,238,833 |
 |
Production
of aromatic carboxylic acids
|
| 72 |
7,237,574 |
 |
Controlled
dispersion multi-phase nozzle and method of
making the same
|
| 73 |
7,235,421 |
 |
System and
method for developing production nano-material
|
| 74 |
7,235,224 |
 |
Process for
preparing fine metal oxide particles
|
| 75 |
7,232,053 |
 |
Seam-welded
air hardenable steel constructions
|
| 76 |
7,229,979 |
 |
Hypoestoxides, derivatives and agonists thereof
for use as stent-coating agents
|
| 77 |
7,226,771 |
 |
Phospholipases, nucleic acids encoding them and
methods for making and using them
|
| 78 |
7,217,750 |
 |
Process for
incorporating one or more materials into a
polymer composition and products produced
thereby |
| 79 |
7,216,830 |
 |
Wing gull
integration nacelle clearance, compact landing
gear stowage, and sonic boom reduction
|
| 80 |
7,216,498 |
 |
Method and
apparatus for determining supercritical pressure
in a heat exchanger
|
| 81 |
7,216,427 |
 |
Surface
treatment of prefinished valve seat inserts
|
| 82 |
7,214,394 |
 |
Policosanol
compositions, extraction from novel sources, and
uses thereof
|
| 83 |
7,211,553 |
 |
Processing
of substrates with dense fluids comprising
acetylenic diols and/or alcohols
|
| 84 |
7,211,145 |
 |
Substrate
processing apparatus and substrate processing
method |
| 85 |
7,208,181 |
 |
Isolation of
polyphenolic compounds from fruits or vegetables
utilizing sub-critical water extraction
|
| 86 |
7,201,804 |
 |
Cleaning of
hydrocarbon-containing materials with critical
and supercritical solents
|
| 87 |
7,201,019 |
 |
Light gas
component separation from a carbon dioxide
mixture |
| 88 |
7,197,876 |
 |
System and
apparatus for power system utilizing wide
temperature range heat sources
|
| 89 |
7,197,404 |
 |
Computation
of radiating particle and wave distributions
using a generalized discrete field constructed
from representative ray sets
|
| 90 |
7,195,676 |
 |
Method for
removal of flux and other residue in dense fluid
systems |
| 91 |
7,193,120 |
 |
Colloid-catalyzed gas transfer in supercritical
phases |
| 92 |
7,193,097 |
 |
Method of
producing a fatty acid ester
|
| 93 |
7,192,995 |
 |
Homogenous
compositions of polymers and crystalline solids
or cross-linking agents and methods of making
the same |
| 94 |
7,192,217 |
 |
Baffle
apparatus |
| 95 |
7,189,350 |
 |
Method of
sterilizing medical instruments
|
| 96 |
7,189,196 |
 |
Method of
separating materials with a concentric tubular
centrifuge
|
| 97 |
7,183,229 |
 |
Semiconductor thin film forming method,
production methods for semiconductor device and
electrooptical device, devices used for these
methods, and semiconductor device and
electrooptical device
|
| 98 |
7,182,861 |
 |
System for
separating electrophotographic carrier
compositions and recycling the compositions
|
| 99 |
7,181,862 |
 |
Sol-gel
process for the production of glassy articles
|
| 100 |
7,178,362 |
 |
Expansion
device arrangement for vapor compression system
|
| 101 |
7,175,886 |
 |
Apparatus and method
for micron and submicron particle formation
|
| 102 |
7,174,713 |
 |
Method for
determination of composition of the gas mixture in a combustion chamber
of an internal combustion engine with exhaust gas recirculation and
correspondingly configured control system for an internal combustion
engine |
| 103 |
7,169,857 |
 |
Homogenous
compositions of fluoropolymers and crystalline solids or cross-linking
agents and methods of making the same
|
| 104 |
7,169,179 |
 |
Drug delivery device
and method for bi-directional drug delivery
|
| 105 |
7,166,753 |
 |
Process for
production of hydrogen and carbonyl compounds by reacting sub- or
super-critical water with alcohols
|
| 106 |
7,166,658 |
 |
Rubber reduction
|
| 107 |
7,164,197 |
 |
Dielectric composite
material |
| 108 |
7,163,989 |
 |
Processes and
apparatus for continuous solution polymerization
|
| 109 |
7,162,661 |
 |
Systematic and random
error detection and recovery within processing stages of an integrated
circuit |
| 110 |
7,160,492 |
 |
Orthopaedic device
for implantation in the body of an animal and method for making the same
|
| 111 |
7,160,380 |
 |
Organic pigment
fine-particle, and method of producing the same
|
| 112 |
7,159,409 |
 |
Method and apparatus
for controlling the load placed on a compressor
|
| 113 |
7,157,886 |
 |
Power converter
method and apparatus having high input power factor and low harmonic
distortion |
| 114 |
7,157,534 |
 |
Polymerization
process for producing copolymers |
| 115 |
7,157,002 |
 |
Process for the
recovery of surfactants |
| 116 |
7,156,925 |
 |
Using supercritical
fluids to clean lenses and monitor defects
|
| 117 |
7,148,387 |
 |
Process for producing
hydroxyl group-containing compound
|
| 118 |
7,147,670 |
 |
Cleaning system
utilizing an organic cleaning solvent and a pressurized fluid solvent
|
| 119 |
7,147,436 |
 |
Turbine engine rotor
retainer |
| 120 |
7,144,498 |
 |
Supercritical
hydrocarbon conversion process |
| 121 |
7,144,461 |
 |
Method of removing
acid component in deteriorated acetate film
|
| 122 |
7,140,197 |
 |
Means and apparatus
for microrefrigeration |
| 123 |
7,131,294 |
 |
Method and apparatus
for control of carbon dioxide gas cooler pressure by use of a capillary
tube |
| 124 |
7,131,291 |
 |
Compression system
for cooling and heating purposes |
| 125 |
7,131,259 |
 |
High density combined
cycle power plant process |
| 126 |
7,129,200 |
 |
Domestic fabric
article refreshment in integrated cleaning and treatment processes
|
| 127 |
7,127,905 |
 |
Vapor compression
system startup method |
| 128 |
7,118,696 |
 |
Method of forming
light dispersing fiber and fiber formed thereby
|
| 129 |
7,114,508 |
 |
Cleaning apparatus
having multiple wash tanks for carbon dioxide dry cleaning and methods
of using same |
| 130 |
7,111,630 |
 |
High pressure
processing apparatus and method |
| 131 |
7,108,867 |
 |
Process for preparing
particles |
| 132 |
7,108,223 |
 |
Missile control
system and method |
| 133 |
7,105,185 |
 |
Kavalactone profile
|
| 134 |
7,101,499 |
 |
Method of and
apparatus for producing pellets from heavy hydrocarbon liquid
|
| 135 |
7,097,715 |
 |
Cleaning system
utilizing an organic cleaning solvent and a pressurized fluid solvent
|
| 136 |
7,096,679 |
 |
Transcritical vapor
compression system and method of operating including refrigerant storage
tank and non-variable expansion device
|
| 137 |
7,091,366 |
 |
Recovery of residual
specialty oil |
| 138 |
7,091,288 |
 |
Polymerization of
vinylidene fluoride (VF.sub.2) in a supercritical fluid medium
|
| 139 |
7,090,871 |
 |
Composition
containing pyrrolizidine-alkaloid-free petasites
|
| 140 |
7,084,269 |
 |
Process for producing
lactam |
| 141 |
7,084,181 |
 |
Method for
decomposing nonmetallic honeycomb panel, and method for recycling the
same |
| 142 |
7,082,355 |
 |
Wake wash severity
monitor for high speed vessels |
| 143 |
7,081,486 |
 |
Method of producing
polymer |
| 144 |
7,081,133 |
 |
Antibiotic treated
implantable medical devices |
| 145 |
7,080,651 |
 |
High pressure
processing apparatus and method |
| 146 |
7,078,731 |
 |
Gallium nitride
crystals and wafers and method of making
|
| 147 |
7,078,359 |
 |
Aerogel composite
with fibrous batting |
| 148 |
RE39,171 |
 |
Simulated wave water
sculpture |
| 149 |
7,074,881 |
 |
Methods of synthesis
of polysuccinimide, copolymers of polysuccinimide and derivatives
thereof |
| 150 |
7,074,369 |
 |
Method and apparatus
for decoupled thermo-catalytic pollution control |
| 151 |
7,072,229 |
 |
Memory system having
fast and slow data reading mechanisms
|
| 152 |
7,070,146 |
 |
Aircraft
thickness/camber control device for low sonic boom
|
| 153 |
7,067,640 |
 |
Cross-linked chiral
compounds and methods of making thereof
|
| 154 |
7,064,834 |
 |
Method for analyzing
impurities in carbon dioxide |
| 155 |
7,063,795 |
 |
Method for starting
up a system for treating waste by hydrothermal oxidation
|
| 156 |
7,063,750 |
 |
Domestic fabric
article refreshment in integrated cleaning and treatment processes
|
| 157 |
7,059,831 |
 |
Turbine engine disk
spacers |
| 158 |
7,059,512 |
 |
Solder alloy material
layer composition, electroconductive and adhesive composition, flux
material layer composition, solder ball transferring sheet, bump and
bump forming process, and semiconductor device
|
| 159 |
7,058,100 |
 |
Systems and methods
for thermal management of diode-pumped solid-state lasers
|
| 160 |
7,057,055 |
 |
Process for preparing
a deuterated or tritiated compound
|
| 161 |
7,056,887 |
 |
Treatment of acute
coronary syndrome with GLP-1 |
| 162 |
7,049,475 |
 |
Organic compound
decomposing method |
| 163 |
7,049,030 |
 |
Battery
|
| 164 |
7,045,642 |
 |
Process for preparing
a deuterated or tritiated compound
|
| 165 |
7,045,622 |
 |
Process for producing
lactam |
| 166 |
7,039,565 |
 |
Method and system for
developing a numerical dynamic sanitary sewer and storm water drainage
simulation model |
| 167 |
7,039,528 |
 |
Method for detecting
leak before rupture in a pipeline |
| 168 |
7,038,000 |
 |
Process for preparing
propylene copolymers |
| 169 |
7,037,524 |
 |
Oral delivery of a
botanical |
| 170 |
7,037,420 |
 |
Anodic oxidation
method and treatment apparatus thereof
|
| 171 |
7,034,092 |
 |
Process for producing
bimodal polyethylene resins |
| 172 |
7,034,084 |
 |
Process and apparatus
for the hydrogenation of polymers under supercritical conditions
|
| 173 |
7,033,985 |
 |
Domestic fabric
article refreshment in integrated cleaning and treatment processes
|
| 174 |
7,033,068 |
 |
Substrate processing
apparatus for processing substrates using dense phase gas and sonic
waves |
| 175 |
7,032,373 |
 |
Device for cooling
coolant in a gas turbine and gas and steam turbine with said device
|
| 176 |
7,029,707 |
 |
Method of producing a
processed kava product having an altered kavalactone distribution and
processed kava products produced using the same
|
| 177 |
7,028,494 |
 |
Defrosting
methodology for heat pump water heating system
|
| 178 |
7,025,992 |
 |
Pharmaceutical
formulations |
| 179 |
7,022,863 |
 |
Production method of
pyrrolidone carboxylic acid and salt thereof
|
| 180 |
7,021,106 |
 |
Apparatus and method
for forming internally ribbed or rifled tubes
|
| 181 |
7,018,902 |
 |
Gate dielectric and
method |
| 182 |
7,009,215 |
 |
Group III-nitride
based resonant cavity light emitting devices fabricated on single
crystal gallium nitride substrates
|
| 183 |
7,008,528 |
 |
Process and system
for continuously extracting oil from solid or liquid oil bearing
material |
| 184 |
7,005,301 |
 |
Piecewise uniform
conduction-like flow channels and method therefor
|
| 185 |
7,003,968 |
 |
Motor vehicle
air-conditioning installation equipped with an electronic control device
|
| 186 |
7,001,620 |
 |
Kavalactone product
|
| 187 |
7,001,581 |
 |
Method for producing
nanocarbon materials |
| 188 |
7,000,870 |
 |
Laminar flow wing for
transonic cruise |
| 189 |
7,000,653 |
 |
High pressure
processing apparatus and high pressure processing method
|
| 190 |
7,000,413 |
 |
Control of
refrigeration system to optimize coefficient of performance
|
| 191 |
6,989,123 |
 |
Methods to produce
gel sheets |
| 192 |
6,978,638 |
 |
Nitrogen rejection
from condensed natural gas |
| 193 |
6,977,767 |
 |
Plasmonic
nanophotonics methods, materials, and apparatuses
|
| 194 |
6,977,520 |
 |
Time-multiplexed
routing in a programmable logic device architecture
|
| 195 |
6,972,271 |
 |
Supported catalyst
systems |
| 196 |
6,968,708 |
 |
Refrigeration system
having variable speed fan |
| 197 |
6,966,874 |
 |
Concentric tubular
centrifuge |
| 198 |
6,964,787 |
 |
Method and system for
reducing microbial burden on a food product
|
| 199 |
6,964,168 |
 |
Advanced heat
recovery and energy conversion systems for power generation and
pollution emissions reduction, and methods of using same
|
| 200 |
6,962,714 |
 |
Critical fluid
antimicrobial compositions and their use and generation
|
| 201 |
6,962,629 |
 |
Method for moving
contaminants from gases |
| 202 |
6,960,672 |
 |
Processes for
producing alkyl ester of fatty acid
|
| 203 |
6,960,618 |
 |
Preparation method
for rigid polyurethane foam |
| 204 |
6,958,123 |
 |
Method for removing a
sacrificial material with a compressed fluid
|
| 205 |
6,958,122 |
 |
High pressure and
high temperature reaction system |
| 206 |
6,956,140 |
 |
Hydrothermal
hydrolysis of halogenated compounds
|
| 207 |
6,955,051 |
 |
Steam generation
apparatus and methods |
| 208 |
6,953,564 |
 |
Method for producing
fullerenes |
| 209 |
6,952,346 |
 |
Etched open
microchannel spray cooling |
| 210 |
6,948,238 |
 |
Method for
dissociating metals from metal compounds
|
| 211 |
6,946,150 |
 |
Pharmaceutical
formulation |
| 212 |
6,944,067 |
 |
Memory system having
fast and slow data reading mechanisms
|
| 213 |
6,943,461 |
 |
All-weather energy
and water production via steam-enhanced vortex tower
|
| 214 |
6,941,854 |
 |
Sliding pairing for
machine parts that are subjected to the action of highly pressurized and
high-temperature steam, preferably for piston-cylinder assemblies of
steam engines |
| 215 |
6,939,837 |
 |
Non-immersive method
for treating or cleaning fabrics using a siloxane lipophilic fluid
|
| 216 |
6,939,651 |
 |
Electrophotographic
photoconductor, and process cartridge and electrophotographic apparatus
using the same |
| 217 |
6,936,843 |
 |
Fixture used to
prepare semiconductor specimens for film adhesion testing
|
| 218 |
6,936,292 |
 |
Deodorized yellow
colorant of safflower |
| 219 |
6,935,592 |
 |
Aircraft lift device
for low sonic boom |
| 220 |
6,932,541 |
 |
Wave forming
apparatus and method |
| 221 |
6,931,372 |
 |
Joint multiple
program coding for digital audio broadcasting and other applications
|
| 222 |
6,929,752 |
 |
Method for treating
waste by hydrothermal oxidation |
| 223 |
6,924,407 |
 |
Pressure-tuned solid
catalyzed heterogeneous chemical reactions
|
| 224 |
6,924,004 |
 |
Apparatus and method
for synthesizing films and coatings by focused particle beam deposition
|
| 225 |
6,923,011 |
 |
Multi-stage vapor
compression system with intermediate pressure vessel
|
| 226 |
6,921,820 |
 |
Method for forming
cellulose |
| 227 |
6,921,420 |
 |
Apparatus and methods
for conserving vapor in a carbon dioxide dry cleaning system
|
| 228 |
6,919,421 |
 |
Methods of synthesis
of polysuccinimide, copolymers of polysuccinimide and derivatives
thereof |
| 229 |
6,913,779 |
 |
Process for the
preparation of accelerated release formulations using compressed fluids
|
| 230 |
6,908,660 |
 |
Shaped body made of
fiber-reinforced composites having a segmented covering layer, its
production and its use |
| 231 |
6,906,423 |
 |
Mask used for
exposing a porous substrate |
| 232 |
6,905,556 |
 |
Method and apparatus
for using surfactants in supercritical fluid processing of wafers
|
| 233 |
6,905,555 |
 |
Methods for
transferring supercritical fluids in microelectronic and other
industrial processes |
| 234 |
6,903,181 |
 |
Methods of synthesis
of polysuccinimide, copolymers of polysuccinimide and derivatives
thereof |
| 235 |
6,902,999 |
 |
Pattern formation
method |
| 236 |
6,901,814 |
 |
Fuselage pitot-static
tube |
| 237 |
6,898,951 |
 |
Washing apparatus
|
| 238 |
6,898,941 |
 |
Supercritical
pressure regulation of vapor compression system by regulation of
expansion machine flowrate |
| 239 |
6,898,936 |
 |
Compression stripping
of flue gas with energy recovery |
| 240 |
6,893,995 |
 |
Supported catalyst
systems |
| 241 |
6,890,660 |
 |
Combustion chamber
with internal jacket made of a ceramic composite material and process
for manufacture |
| 242 |
6,887,992 |
 |
3-cephem derivative
crystal |
| 243 |
6,887,971 |
 |
Synthesis of
polysuccinimide and copoly(succinimide-aspartate) in a supercritical
fluid |
| 244 |
6,887,516 |
 |
Method and apparatus
for applying a powder coating |
| 245 |
6,886,487 |
 |
Thruster apparatus
and method for reducing fluid-induced motions of and stresses within an
offshore platform |
| 246 |
6,884,911 |
 |
Material processing
by repeated solvent expansion-contraction
|
| 247 |
6,884,900 |
 |
Method for producing
fatty acid alcohol ester |
| 248 |
6,884,737 |
 |
Method and apparatus
for precursor delivery utilizing the melting point depression of solid
deposition precursors in the presence of supercritical fluids
|
| 249 |
6,881,800 |
 |
Processes and
apparatus for continuous solution polymerization
|
| 250 |
6,881,700 |
 |
Dealuminized catalyst
carrier, method of production, and method for hydrating C2 or C3 olefins
with water |
| 251 |
6,881,699 |
 |
Method for producing
a dealuminized catalyst support |
| 252 |
6,880,560 |
 |
Substrate processing
apparatus for processing substrates using dense phase gas and sonic
waves |
| 253 |
6,880,326 |
 |
High regression rate
hybrid rocket propellants and method of selecting
|
| 254 |
6,878,466 |
 |
Method for improving
the reliability of brittle materials through the creation of a threshold
strength |
| 255 |
6,874,513 |
 |
High pressure
processing apparatus |
| 256 |
6,858,179 |
 |
Process for producing
sterile water for injection from potable water
|
| 257 |
6,857,437 |
 |
Automated dense phase
fluid cleaning system |
| 258 |
6,849,678 |
 |
Polymerization,
compatibilized blending, and particle size control of powder coatings in
a supercritical fluid |
| 259 |
6,848,269 |
 |
Process and apparatus
for the recovery of krypton and/or xenon
|
| 260 |
6,846,562 |
 |
Method of forming
light dispersing fiber and fiber formed thereby
|
| 261 |
6,845,900 |
 |
Methods for producing
weld joints having thermally enhanced heat-affected-zones with excellent
fracture toughness |
| 262 |
6,844,561 |
 |
Rotating aperture
system |
| 263 |
6,843,193 |
 |
Transonic hull and
hydrofield (part III) |
| 264 |
6,841,031 |
 |
Substrate processing
apparatus equipping with high-pressure processing unit
|
| 265 |
6,828,292 |
 |
Domestic fabric
article refreshment in integrated cleaning and treatment processes
|
| 266 |
6,825,588 |
 |
Uninterruptible power
supply using a high speed cylinder flywheel
|
| 267 |
6,825,260 |
 |
Nanoporous
interpenetrating organic-inorganic networks
|
| 268 |
6,823,880 |
 |
High pressure
processing apparatus and high pressure processing method
|
| 269 |
6,821,554 |
 |
Polyol-based method
for forming thin film aerogels on semiconductor substrates
|
| 270 |
6,821,485 |
 |
Method and structure
for microfluidic flow guiding |
| 271 |
6,821,481 |
 |
Continuous processing
method and continuous processing apparatus for liquid-form substance,
and liquid-form substance processed thereby
|
| 272 |
6,821,429 |
 |
Method and device for
capturing fine particles by trapping in a solid mixture of carbon
dioxide snow type |
| 273 |
6,821,413 |
 |
Method and apparatus
for continuous separation and reaction using supercritical fluid
|
| 274 |
6,818,072 |
 |
High-strength
heat-resistant steel, process for producing the same, and process for
producing high-strength heat-resistant pipe
|
| 275 |
6,818,021 |
 |
Domestic fabric
article refreshment in integrated cleaning and treatment processes
|
| 276 |
6,817,193 |
 |
Method for operating
a refrigerant circuit, method for operating a motor vehicle driving
engine, and refrigerant circuit |
| 277 |
6,813,895 |
 |
Supercritical
pressure regulation of vapor compression system by regulation of
adaptive control |
| 278 |
6,805,801 |
 |
Method and apparatus
to remove additives and contaminants from a supercritical processing
solution |
| 279 |
6,801,593 |
 |
Subcritical
reactivity measurement method |
| 280 |
6,800,773 |
 |
Chemical process
|
| 281 |
6,800,316 |
 |
Method for
fractionating cooking oil |
| 282 |
6,800,272 |
 |
Process for the
preparation of ZSM-5 catalyst |
| 283 |
6,799,587 |
 |
Apparatus for
contaminant removal using natural convection flow and changes in
solubility concentrations by temperature
|
| 284 |
6,797,738 |
 |
Open pore
biodegradable matrices |
| 285 |
6,796,275 |
 |
Method and apparatus
for calculating minimum valve lift for internal combustion engines
|
| 286 |
6,796,137 |
 |
Air conditioning
system comprising an electronic control device
|
| 287 |
6,795,991 |
 |
Apparatus for
conserving vapor in a carbon dioxide dry cleaning system
|
| 288 |
6,795,780 |
 |
Fluid energy pulse
test system--transient, ramp, steady state tests
|
| 289 |
6,794,544 |
 |
Method for the
preparation of tetrahydrobenzothiepines
|
| 290 |
6,794,522 |
 |
Process for preparing
a deuterated or tritiated compound
|
| 291 |
6,793,975 |
 |
Methods of chemical
vapor deposition and powder formation
|
| 292 |
6,793,793 |
 |
Electrochemical
treating method such as electroplating and electrochemical reaction
device therefor |
| 293 |
6,792,759 |
 |
High density combined
cycle power plant process |
| 294 |
6,790,790 |
 |
High modulus filler
for low k materials |
| 295 |
6,790,707 |
 |
Method of preparing a
sample of a semiconductor structure for adhesion testing
|
| 296 |
6,774,421 |
 |
Gapped-plate
capacitor |
| 297 |
6,774,262 |
 |
Method of novel
noncatalytic organic synthesis |
| 298 |
6,773,581 |
 |
System and method for
solids transport in hydrothermal processes
|
| 299 |
6,766,810 |
 |
Methods and apparatus
to control pressure in a supercritical fluid reactor
|
| 300 |
6,765,113 |
 |
Production of
aromatic carboxylic acids |
| 301 |
6,764,552 |
 |
Supercritical
solutions for cleaning photoresist and post-etch residue from low-k
materials |
| 302 |
6,763,271 |
 |
Tracking sustained
chaos |
| 303 |
6,755,871 |
 |
Cleaning system
utilizing an organic cleaning solvent and a pressurized fluid solvent
|
| 304 |
6,751,959 |
 |
Simple and compact
low-temperature power cycle |
| 305 |
6,750,336 |
 |
Method of production
of lactam |
| 306 |
6,750,062 |
 |
Method and device for
studying the effect of a supercritical fluid on the transition of a
material from one condensed phase to the other and their application in
the case of a polymer material |
| 307 |
6,749,816 |
 |
High-pressure
treatment apparatus, feeding method thereto and protection method
thereof |
| 308 |
6,746,788 |
 |
Concentration cells
utilizing external fields |
| 309 |
6,746,695 |
 |
Pharmaceutical
preparations of bioactive substances extracted from natural sources
|
| 310 |
6,741,669 |
 |
Neutron absorber
systems and method for absorbing neutrons
|
| 311 |
6,740,785 |
 |
Catalytic oxidation
of organic substrates by transition metal complexes in organic solvent
media expanded by supercritical or subcritical carbon dioxide
|
| 312 |
6,740,416 |
 |
Aerogel substrate and
method for preparing the same |
| 313 |
6,740,414 |
 |
Magnetic recording
media and magnetic recording apparatus used thereof
|
| 314 |
6,740,216 |
 |
Potentiometric sensor
for wellbore applications |
| 315 |
6,739,141 |
 |
Supercritical
pressure regulation of vapor compression system by use of gas cooler
fluid pumping device |
| 316 |
6,738,446 |
 |
System and method for
radioactive waste destruction |
| 317 |
6,737,254 |
 |
Supercritical
extraction of taxanes |
| 318 |
6,736,859 |
 |
Cleaning system
utilizing an organic cleaning solvent and a pressurized fluid solvent
|
| 319 |
6,733,586 |
 |
High throughput
non-photochemical laser induced nucleation
|
| 320 |
6,730,370 |
 |
Method and apparatus
for processing materials by applying a controlled succession of thermal
spikes or shockwaves through a growth medium
|
| 321 |
6,728,092 |
 |
Formation of thin
film capacitors |
| 322 |
6,723,363 |
 |
Coating foods and
pharmaceuticals with an edible polymer using carbon dioxide
|
| 323 |
6,718,915 |
 |
Horizontal spiral
tube boiler convection pass enclosure design
|
| 324 |
6,717,009 |
 |
Method for making
high-purity naphthalenedicarboxylic acid
|
| 325 |
6,716,663 |
 |
Method for removing
foreign matter, method for forming film, semiconductor device and film
forming apparatus |
| 326 |
6,716,107 |
 |
Containerless sheet
flow water ride |
| 327 |
6,715,450 |
 |
Fossil-fuel fired
continuous-flow steam generator |
| 328 |
6,713,316 |
 |
Method for removing
foreign matter, method for forming film, semiconductor device and film
forming apparatus |
| 329 |
6,712,973 |
 |
Process and device
for separation with variable-length chromatographic zones
|
| 330 |
6,712,081 |
 |
Pressure processing
device |
| 331 |
6,709,602 |
 |
Process for
hydrothermal treatment of materials
|
| 332 |
6,709,595 |
 |
Method and
installation for setting in adsorbed state on a porous support active
compounds contained in a product |
| 333 |
6,708,513 |
 |
CO2-module for
cooling and heating |
| 334 |
6,707,205 |
 |
High-speed,
high-power rotary electrodynamic machine with dual rotors
|
| 335 |
6,706,689 |
 |
Treatment of acute
coronary syndrome with GLP-1 |
| 336 |
6,705,103 |
 |
Device and method for
cooling |
| 337 |
6,701,721 |
 |
Stirling engine
driven heat pump with fluid interconnection
|
| 338 |
6,699,808 |
 |
High-solids SiO2
dispersions, process for producing them, and their use
|
| 339 |
6,698,234 |
 |
Method for increasing
efficiency of a vapor compression system by evaporator heating
|
| 340 |
6,698,214 |
 |
Method of
refrigeration with enhanced cooling capacity and efficiency
|
| 341 |
6,694,763 |
 |
Method for operating
a transcritical refrigeration system
|
| 342 |
6,693,222 |
 |
Process for splitting
water-soluble ethers |
| 343 |
6,691,536 |
 |
Washing apparatus
|
| 344 |
6,691,430 |
 |
High-pressure drying
apparatus, high-pressure drying method and substrate processing
apparatus |
| 345 |
6,689,311 |
 |
Method and apparatus
for manufacturing sinter, method for measuring concentration of
plasticizer, evaluation method, and evaluation apparatus
|
| 346 |
6,685,394 |
 |
Partial shroud with
perforating for VIV suppression, and method of using
|
| 347 |
6,684,643 |
 |
Process for the
operation of a gas turbine plant |
| 348 |
6,684,624 |
 |
High regression rate
hybrid rocket propellants |
| 349 |
6,680,554 |
 |
Aircraft,
particularly a helicopter, transmission system
|
| 350 |
6,673,234 |
 |
Combined process of
low degree solvent deasphalting and delayed coking
|
| 351 |
6,673,171 |
 |
Medium carbon steel
sheet and strip having enhanced uniform elongation and method for
production thereof |
| 352 |
6,672,732 |
 |
Micromechanical
oscillating device |
| 353 |
6,670,407 |
 |
Biofunctional
polymers prepared in supercritical fluid
|
| 354 |
6,667,019 |
 |
Rare earth oxide
fluoride nanoparticles and hydrothermal method for forming nanoparticles
|
| 355 |
6,666,074 |
 |
Apparatus for
conducting high-temperature liquid chromatography analysis
|
| 356 |
6,666,050 |
 |
Apparatus for
conserving vapor in a carbon dioxide dry cleaning system
|
| 357 |
6,660,176 |
 |
Molecular imprinting
of small particles, and production of small particles from solid state
reactants |
| 358 |
6,658,888 |
 |
Method for increasing
efficiency of a vapor compression system by compressor cooling
|
| 359 |
6,656,436 |
 |
Device for
transforming chemical structures in a fluid comprising a solvent and
salts by ultrasonic action |
| 360 |
6,649,776 |
 |
Methylenelactone
synthesis in supercritical fluids |
| 361 |
6,649,516 |
 |
Method for
manufacturing a composite member from a porous substrate by selectively
infiltrating conductive material into the substrate to form via and
wiring regions |
| 362 |
6,649,091 |
 |
Electrically
conducting ruthenium dioxide aerogel composite
|
| 363 |
6,649,062 |
 |
Fluid-membrane
separation |
| 364 |
6,647,742 |
 |
Expander driven motor
for auxiliary machinery |
| 365 |
6,642,407 |
 |
Production,
purification and polymerization of aromatic dicarboxylic acids
|
| 366 |
6,642,330 |
 |
Supercritical fluid
pressure sensitive adhesive polymers and their preparation
|
| 367 |
6,641,678 |
 |
Methods for cleaning
microelectronic structures with aqueous carbon dioxide systems
|
| 368 |
6,638,749 |
 |
Carbon dioxide
soluble surfactant having two fluoroether CO2-philic tail groups and a
head group |
| 369 |
6,634,576 |
 |
Milled particles
|
| 370 |
6,632,878 |
 |
Production process
for polymers with hydroxyl groups and polymers obtained by the process
|
| 371 |
6,630,761 |
 |
Combination
mechanical and magnetic support for a flywheel power supply
|
| 372 |
6,628,002 |
 |
Heat transfer system
with supracritical fluid |
| 373 |
6,627,246 |
 |
Process for coating
stents and other medical devices using super-critical carbon dioxide
|
| 374 |
6,624,299 |
 |
Process for producing
cellulose acetate |
| 375 |
6,624,092 |
 |
Method for forming
low dielectric constant insulating layer with foamed structure
|
| 376 |
6,620,559 |
 |
Photomask, method of
lithographically structuring a photoresist layer with the photomask, and
method of producing magnetic memory elements
|
| 377 |
6,619,368 |
 |
Method for imaging
inclusions in investment castings |
| 378 |
6,619,041 |
 |
Steam generation
apparatus and methods |
| 379 |
6,616,849 |
 |
Method of and system
for continuously processing liquid materials, and the product processed
thereby |
| 380 |
6,615,587 |
 |
Combustion device and
method for burning a fuel |
| 381 |
6,613,945 |
 |
Resolution method for
a racemic mixture of aldehydes |
| 382 |
6,613,358 |
 |
Sustained-release
composition including amorphous polymer
|
| 383 |
6,613,157 |
 |
Methods for removing
particles from microelectronic structures
|
| 384 |
6,610,251 |
 |
Method of sterilizing
medical instruments |
| 385 |
6,608,878 |
 |
Method and device for
monitoring the power rise during startup of a nuclear reactor (diversitary
excursion monitoring) |
| 386 |
6,606,848 |
 |
High power density
combined cycle power plant system |
| 387 |
6,602,545 |
 |
Method of directly
making rapid prototype tooling having free-form shape
|
| 388 |
6,602,351 |
 |
Methods for the
control of contaminants following carbon dioxide cleaning of
microelectronic structures |
| 389 |
6,601,438 |
 |
Apparatus for
conducting high-temperature liquid chromotography analysis
|
| 390 |
6,598,358 |
 |
Use of aerogels for
deadening structure-borne and/or impact sounds
|
| 391 |
6,596,093 |
 |
Methods for cleaning
microelectronic structures with cyclical phase modulation
|
| 392 |
6,596,077 |
 |
Controlled nucleation
of protein crystals |
| 393 |
6,592,938 |
 |
Method for coating
particles |
| 394 |
6,590,053 |
 |
Supercritical fluid
pressure sensitive adhesive polymers and their preparation
|
| 395 |
6,589,592 |
 |
Methods of coating
articles using a densified coating system
|
| 396 |
6,589,355 |
 |
Cleaning processes
using hydrofluorocarbon and/or hydrochlorofluorocarbon compounds
|
| 397 |
6,588,285 |
 |
Fuselage pitot-static
tube and the aerodynamic profile of its strut
|
| 398 |
6,586,434 |
 |
Method for the
preparation of tetrahydrobenzothiepines
|
| 399 |
6,585,958 |
 |
Medicinal aerosol
formulations |
| 400 |
6,585,890 |
 |
Process for producing
sterile water for injection from potable water |
| 401 |
6,582,666 |
 |
Apparatus for high
flux photocatalytic pollution control using a rotating fluidized bed
reactor |
| 402 |
6,582,508 |
 |
Process for fine
division of organic pigments |
| 403 |
6,577,697 |
 |
Field analysis of
geological samples using delayed neutron activation analysis
|
| 404 |
6,576,185 |
 |
System and method for
hydrothermal reactions-three layer liner
|
| 405 |
6,576,066 |
 |
Supercritical drying
method and supercritical drying apparatus
|
| 406 |
6,574,138 |
 |
Memory cell
configuration and method for operating the configuration
|
| 407 |
6,572,759 |
 |
Method and apparatus
for treating aqueous medium |
| 408 |
6,569,480 |
 |
Liquefied gas
extraction process |
| 409 |
6,569,245 |
 |
Method and apparatus
for applying a powder coating |
| 410 |
6,568,199 |
 |
Method for optimizing
coefficient of performance in a transcritical vapor compression system
|
| 411 |
6,565,682 |
 |
Process for producing
high-strength heat-resistant pipe |
| 412 |
6,564,567 |
 |
Method and device for
heating and cooling a compartment of a motor vehicle
|
| 413 |
6,562,914 |
 |
Process for making
propylene homo or copolymers |
| 414 |
6,562,898 |
 |
Resin composition and
manufacturing method therefor |
| 415 |
6,562,605 |
 |
Extraction of water
soluble biomaterials from fluids using a carbon dioxide/surfactant
mixture |
| 416 |
6,562,146 |
 |
Processes for
cleaning and drying microelectronic structures using liquid or
supercritical carbon dioxide |
| 417 |
6,558,622 |
 |
Sub-critical fluid
cleaning and antimicrobial decontamination system and process
|
| 418 |
6,558,432 |
 |
Cleaning system
utilizing an organic cleaning solvent and a pressurized fluid solvent
|
| 419 |
6,554,774 |
 |
Method and apparatus
for assessing hemodynamic properties within the circulatory system of a
living subject |
| 420 |
6,551,626 |
 |
Composition
containing pyrrolizidine-alkaloid-free petasites
|
| 421 |
6,551,561 |
 |
Apparatus for
decoupled thermo-photocatalytic pollution control
|
| 422 |
6,551,517 |
 |
Method for
transforming chemical structures in a fluid under pressure and in high
temperature |
| 423 |
6,550,974 |
 |
Bearing assembly for
a helicopter rear transmission shaft
|
| 424 |
6,548,647 |
 |
Process for preparing
azo colorants |
| 425 |
6,541,278 |
 |
Method of forming
film for semiconductor device with supercritical fluid
|
| 426 |
6,539,536 |
 |
Electronic design
automation system and methods utilizing groups of multiple cells having
loop-back connections for modeling port electrical characteristics
|
| 427 |
6,537,395 |
 |
Process for producing
gray cast iron for use in high speed machining with cubic boron nitride
and silicon nitride tools |
| 428 |
6,537,364 |
 |
Process for fine
division of organic pigments by precipitation
|
| 429 |
6,536,380 |
 |
Fossil-fuel heated
steam generator, comprising dentrification device for heating gas
|
| 430 |
6,532,550 |
 |
Process protection
system |
| 431 |
6,531,035 |
 |
Apparatus and method
for low flux photocatalytic pollution control
|
| 432 |
6,528,669 |
 |
Recovery of
polyunsaturated fatty acids from urea adducts
|
| 433 |
RE38,001 |
 |
Cleaning with liquid
gases |
| 434 |
6,526,115 |
 |
Supercritical-pressure water cooled reactor and power generation plant
|
| 435 |
6,520,767 |
 |
Fuel delivery system
for combusting fuel mixtures |
| 436 |
6,519,926 |
 |
Hydrothermal
conversion and separation |
| 437 |
6,519,847 |
 |
Surface treatment of
prefinished valve seat inserts |
| 438 |
6,517,840 |
 |
Production of a
product enriched in isoflavone values from natural sources
|
| 439 |
6,517,752 |
 |
Surface reforming
method for plastic molded product |
| 440 |
6,517,694 |
 |
Yttria-stabilized
zirconia membrane electrode |
| 441 |
6,514,556 |
 |
Method and
composition for washing poultry during processing
|
| 442 |
6,514,454 |
 |
Sol-gel process using
porous mold |
| 443 |
6,510,739 |
 |
Apparatus for
continuously monitoring liquid level conditions in a liquid-vapor
separating device |
| 444 |
6,507,191 |
 |
NMR cell system for
supercritical fluid measurements and high-pressure cell for NMR
|
| 445 |
6,505,807 |
 |
Blower motor with
annular damping element |
| 446 |
6,503,360 |
 |
Etching method and
apparatus |
| 447 |
6,500,350 |
 |
Formation of thin
film resistors |
| 448 |
6,499,463 |
 |
Dual fuel source
diesel engine |
| 449 |
6,499,440 |
 |
Fossil-fired steam
generator |
| 450 |
6,499,313 |
 |
Process and apparatus
for generating high-purity nitrogen by low-temperature fractionation of
air |
| 451 |
6,496,757 |
 |
Nonlinear contingency
screening for voltage collapse |
| 452 |
6,495,190 |
 |
Cellulose-containing
composite |
| 453 |
6,494,045 |
 |
High density combined
cycle power plant process |
| 454 |
6,491,589 |
 |
Mobile water ride
having sluice slide-over cover |
| 455 |
6,488,849 |
 |
Rotating device for
continuously filtering a liquid such as a solution containing a
precipitate |
| 456 |
6,487,994 |
 |
Sub-critical
water-fuel composition and combustion system
|
| 457 |
6,486,078 |
 |
Super critical drying
of low k materials |
| 458 |
6,485,174 |
 |
Attachable heat flux
measuring device |
| 459 |
6,484,519 |
 |
Motor vehicle
air-conditioning system and a method for operating a motor vehicle air
conditioning system |
| 460 |
6,482,390 |
 |
Aerosol formulations
and method |
| 461 |
6,481,386 |
 |
Fossil-fired
continuous-flow steam generator |
| 462 |
6,481,247 |
 |
Cleaning method and
apparatus with dense phase fluid |
| 463 |
6,478,955 |
 |
Membrane magnetic
separating apparatus |
| 464 |
6,475,561 |
 |
Method of producing
silicon tetrachloride-based and organically modified aerogels
|
| 465 |
6,475,524 |
 |
Composition of matter
|
| 466 |
6,475,403 |
 |
Etching method and
apparatus |
| 467 |
6,467,156 |
 |
Method and apparatus
for preparing electrochemical cells
|
| 468 |
6,464,861 |
 |
Apparatus for
treating flora and fauna waste with hydrothermal reaction
|
| 469 |
6,464,797 |
 |
Method of separating
electrophotographic carrier compositions and recycling the compositions
|
| 470 |
6,462,230 |
 |
Method of and
apparatus for decomposing wastes |
| 471 |
6,461,642 |
 |
Crystallization using
supercritical or subcritical fluids
|
| 472 |
6,461,591 |
 |
Medical aerosol
formulations |
| 473 |
6,460,012 |
 |
Nonlinear structural
crack growth monitoring |
| 474 |
6,458,407 |
 |
Process for producing
essential oil by subcritical or supercritical water treatment
|
| 475 |
6,455,010 |
 |
Apparatus and method
for pressurizing a propylene polymerization reactor
|
| 476 |
6,451,375 |
 |
Process for
depositing a film on a nanometer structure
|
| 477 |
6,450,780 |
 |
Method for generating
over-pressure gas |
| 478 |
6,450,680 |
 |
Apparatus for mixing
powder |
| 479 |
6,448,454 |
 |
Catalytic oxidation
of organic substrates by transition metal complexes in organic solvent
media expanded by supercritical or subcritical carbon dioxide
|
| 480 |
6,446,580 |
 |
Fossil fuel-fired
continuous-flow steam generator |
| 481 |
6,444,772 |
 |
Supercritical fluid
pressure sensitive adhesive polymers and their preparation
|
| 482 |
6,442,226 |
 |
Accelerator-driven
transmutation of spent fuel elements
|
| 483 |
6,436,991 |
 |
Composition and
method containing products extracted from Commiphora sp. for prevention
and treatment of abnormal cell growth and proliferation in inflammation,
neoplasia and cardiovascular disease
|
| 484 |
6,433,993 |
 |
Formation of thin
film capacitors |
| 485 |
6,430,950 |
 |
Expansion element and
a valve unit usable therefor |
| 486 |
6,427,544 |
 |
Environmentally
friendly ultra-high sensitivity liquid penetrant inspection process and
system |
| 487 |
6,427,308 |
 |
Support assembly for
a rotating shaft |
| 488 |
6,425,568 |
 |
Support assembly for
a rotating shaft |
| 489 |
6,416,682 |
 |
Method of producing
minerals under near-critical, critical and supercritical conditions
|
| 490 |
6,416,624 |
 |
Spray application of
an additive composition to sheet materials
|
| 491 |
6,415,998 |
 |
Circuit grinding
apparatus with high pressure roller mill and sifter
|
| 492 |
6,414,050 |
 |
Biofunctional
polymers prepared in supercritical fluid
|
| 493 |
6,413,419 |
 |
Process and device
for separation with variable-length chromatographic
|
| 494 |
6,410,808 |
 |
Method for producing
a fluoroalcohol |
| 495 |
6,407,143 |
 |
Method and solvent
composition for regenerating an ion exchange resin
|
| 496 |
RE37,733 |
 |
Method for settling
of suspensions with use of seepage force and vibrations
|
| 497 |
6,403,544 |
 |
Composition and
method for removing photoresist materials from electronic components
|
| 498 |
6,403,126 |
 |
Cannabinoid
extraction method |
| 499 |
6,397,421 |
 |
Methods and apparatus
for conserving vapor and collecting liquid carbon dioxide for carbon
dioxide dry cleaning |
| 500 |
6,396,387 |
 |
Resistors for
electronic packaging |
| 501 |
6,395,661 |
 |
Sintered silicon
nitride, components made therewith, specially valves, methods for the
production and use thereof |
| 502 |
6,395,343 |
 |
Durable thermal
barrier coating |
| 503 |
6,395,107 |
 |
Cast iron for use in
high speed machining with cubic boron nitride and silicon nitride tools
|
| 504 |
6,391,607 |
 |
Human DNase I
hyperactive variants |
| 505 |
6,381,829 |
 |
Method for making an
improved main shaft for a coal pulverizer by employing a ceramic coating
thereon |
| 506 |
6,375,839 |
 |
Process and device
for separation with variable-length chromatographic zones
|
| 507 |
6,374,618 |
 |
Cryogenic fluid
supply from supercritical storage system
|
| 508 |
6,368,074 |
 |
Piston type
compressor |
| 509 |
6,367,491 |
 |
Apparatus for
contaminant removal using natural convection flow and changes in
solubility concentration by temperature
|
| 510 |
6,367,319 |
 |
Method of controlling
an internal combustion engine in dependence on an exhaust gas pressure
|
| 511 |
6,366,844 |
 |
Method and device for
limiting transversal acceleration in a motor vehicle
|
| 512 |
6,365,638 |
 |
Process for
preparation of hydrophilic or partially hydrophilic inorganic aerogels
|
| 513 |
6,362,118 |
 |
Method of making
stabilized negative thermal expansion optical waveguide substrate and a
glass-ceramic substrate |
| 514 |
6,361,902 |
 |
Method for removing
acidic impurities in a solid electrolyte
|
| 515 |
6,357,029 |
 |
Joint multiple
program error concealment for digital audio broadcasting and other
applications |
| 516 |
6,355,741 |
 |
Process for producing
polyolefins |
| 517 |
6,355,072 |
 |
Cleaning system
utilizing an organic cleaning solvent and a pressurized fluid solvent
|
| 518 |
6,352,644 |
 |
Method of
manipulating the chemical properties of water to improve the
effectiveness of a desired process
|
| 519 |
6,352,065 |
 |
Method and device for
determining the gas intake in an internal combustion engine
|
| 520 |
6,350,890 |
 |
Method for obtaining
fatty acids from biomass by combined in/situ extraction, reaction and
chromatography using compressed gases
|
| 521 |
6,348,343 |
 |
Human DNase I
variants |
| 522 |
6,347,927 |
 |
Piston-type
compressor with bolted separating wall
|
| 523 |
6,347,758 |
 |
Strain relief main
shaft |
| 524 |
6,342,592 |
 |
Chiral compounds,
their synthesis and use as a support
|
| 525 |
6,342,341 |
 |
Fragmentable electron
donor compounds used in conjunction with epitaxially sensitized silver
halide emulsions |
| 526 |
6,342,128 |
 |
Method and apparatus
for decoupled thermo-photocatalytic pollution control
|
| 527 |
6,340,722 |
 |
Polymerization,
compatibilized blending, and particle size control of powder coatings in
a supercritical fluid |
| 528 |
6,336,429 |
 |
Drumless natural
circulation boiler |
| 529 |
6,334,936 |
 |
Method for decoupled
thermo-catalytic pollution control
|
| 530 |
6,332,975 |
 |
Anode grade coke
production |
| 531 |
6,332,342 |
 |
Methods for carbon
dioxide dry cleaning with integrated distribution
|
| 532 |
6,331,320 |
 |
Process for producing
aromatic compounds by supercritical water treatment
|
| 533 |
6,329,899 |
 |
Formation of thin
film resistors |
| 534 |
6,326,504 |
 |
Procedure to extract
natural products |
| 535 |
6,319,137 |
 |
Containerless sheet
flow water ride |
| 536 |
6,316,646 |
 |
Process for the
continuous catalytic transformation of organic compounds
|
| 537 |
6,316,377 |
 |
Rare earth oxide
fluoride nanoparticles and hydrothermal method for forming nanoparticles
|
| 538 |
6,316,326 |
 |
Gapped-plate
capacitor |
| 539 |
6,315,942 |
 |
System for delivery
of gas-enriched fluids |
| 540 |
6,315,870 |
 |
Method for high flux
photocatalytic pollution control |
| 541 |
6,312,529 |
 |
Steel compositions
and methods of processing for producing cold-formed and carburized
components with fine-grained microstructures
|
| 542 |
6,309,611 |
 |
Apparatus for low
flux photocatalytic pollution control
|
| 543 |
6,306,215 |
 |
Apparatus for coating
current collectors |
| 544 |
6,302,194 |
 |
Pipe with ribs on its
inner surface forming a multiple thread and steam generator for using
the pipe |
| 545 |
6,299,705 |
 |
High-strength
heat-resistant steel and process for producing high-strength
heat-resistant steel |
| 546 |
6,298,674 |
 |
Method for operating
a subcritically and transcritically operated vehicle air conditioner
|
| 547 |
6,295,678 |
 |
Method for
pre-balancing a rotating drum having a temporarily shifting unbalance
|
| 548 |
6,294,131 |
 |
Heat resistant steel
|
| 549 |
6,290,880 |
 |
Electrically
conducting ruthenium dioxide-aerogel composite
|
| 550 |
6,288,289 |
 |
Integrated effluent
treatment process for nitroaromatic manufacture |
| 551 |
6,287,304 |
 |
Interstitial
cauterization of tissue volumes with electrosurgically deployed
electrodes |
| 552 |
6,284,200 |
 |
Flushing apparatus in
a simulated mobile bed adsorption unit comprising two fluid distribution
lines, and the use thereof |
| 553 |
6,283,138 |
 |
Pressure relief valve
monitoring device |
| 554 |
6,280,744 |
 |
Use of inorganic
aerogels in pharmacy |
| 555 |
6,277,543 |
 |
Method for forming
features using frequency doubling hybrid resist and device formed
thereby |
| 556 |
6,277,329 |
 |
Dissolved hydrogen
analyzer |
| 557 |
6,277,327 |
 |
Method for the
anticorrosive treatment of waste plastics treating equipment
|
| 558 |
6,277,216 |
 |
Heat-treated steels
with optimized toughness and method thereof
|
| 559 |
6,276,153 |
 |
Method and device for
heating and cooling a compartment of a motor vehicle
|
| 560 |
6,273,921 |
 |
Battery fabrication
method using supercritical carbon dioxide
|
| 561 |
6,272,864 |
 |
Combustor for a gas
turbine |
| 562 |
6,270,835 |
 |
Formation of this
film capacitors |
| 563 |
6,270,026 |
 |
Strain relief main
shaft assembly |
| 564 |
6,268,676 |
 |
Support assembly for
a rotating shaft |
| 565 |
6,260,369 |
 |
Flow control valve
for a variable displacement refrigerant compressor
|
| 566 |
6,258,940 |
 |
Method for producing
erythrose |
| 567 |
6,258,910 |
 |
Polymerizing vinyl
chloride in carbon dioxide |
| 568 |
6,257,020 |
 |
Process for the
cryogenic separation of gases from air
|
| 569 |
RE37,254 |
 |
Track link for
tracked crawlers |
| 570 |
6,256,363 |
 |
Storage/transport
container for spent nuclear-fuel elements
|
| 571 |
6,255,529 |
 |
Method of and
apparatus for decomposing wastes |
| 572 |
6,251,286 |
 |
Accumulating
automatic skimmer |
| 573 |
6,248,910 |
 |
Process for
extracting oil from oil-bearing naturally occurring organic materials
|
| 574 |
6,248,136 |
 |
Methods for carbon
dioxide dry cleaning with integrated distribution
|
| 575 |
6,245,488 |
 |
Method for forming
features using frequency doubling hybrid resist and device formed
thereby |
| 576 |
6,242,038 |
 |
Readily-dispersible
lipidic hop extract for imparting hoppy aroma and flavor to beer
|
| 577 |
6,241,802 |
 |
Method for delivery
of gas-enriched fluids |
| 578 |
6,240,883 |
 |
Sub-critical
water-fuel composition and combustion system
|
| 579 |
6,240,859 |
 |
Cement,
reduced-carbon ash and controlled mineral formation using sub- and
supercritical high-velocity free-jet expansion into fuel-fired combustor
fireballs |
| 580 |
6,239,067 |
 |
Process and apparatus
for the production of activated carbon
|
| 581 |
6,233,298 |
 |
Apparatus for
transmutation of nuclear reactor waste
|
| 582 |
6,231,314 |
 |
Variable displacement
compressor |
| 583 |
6,230,480 |
 |
High power density
combined cycle power plant |
| 584 |
6,228,394 |
 |
Supercritical fluid
extraction of mould lubricant from hard shell capsules
|
| 585 |
6,228,346 |
 |
Propellant mixtures
and aerosols for micronizing medicaments with compressed gas
|
| 586 |
6,227,814 |
 |
Reciprocating type
refrigerant compressor with an improved internal sealing unit
|
| 587 |
6,225,483 |
 |
Cold solvent
extraction process for extracting oil from oil-bearing materials
|
| 588 |
6,223,534 |
 |
Engine-braking
arrangement for an internal combustion engine with an exhaust-gas
turbocharger |
| 589 |
6,221,435 |
 |
Method for the spray
application of polymeric-containing liquid coating compositions using
subcritical compressed fluids under choked flow spraying conditions
|
| 590 |
6,221,181 |
 |
Coating composition
for high temperature protection |
| 591 |
6,216,063 |
 |
On-line .mu. method
for robust flutter prediction in expanding a safe flight envelope for an
aircraft model under flight test |
| 592 |
6,214,944 |
 |
Method for
pressurizing a propylene polymerization reactor
|
| 593 |
6,214,893 |
 |
Polyester
decomposition process and polyester monomerization system
|
| 594 |
6,213,884 |
 |
Case hardened
self-drilling, self-tapping, self-piercing fasteners and process for
making the same |
| 595 |
6,213,710 |
 |
Method and apparatus
for thrust compensation on a turbomachine
|
| 596 |
6,210,751 |
 |
Process for preparing
organically modified aerogels in which the salts formed are precipitated
out |
| 597 |
6,210,592 |
 |
Deposition of
resistor materials directly on insulating substrates
|
| 598 |
6,208,234 |
 |
Resistors for
electronic packaging |
| 599 |
6,207,522 |
 |
Formation of thin
film capacitors |
| 600 |
6,204,401 |
 |
Purifying
polyunsaturated fatty acid glycerides |
Worldwide Patent Subcritical
[TW270555B]Method of
manufacturing polymer/
2007-01-11
The method of manufacturing polymer according to this invention is characterized
by photopolymerizing at least one kind of polymerization precursor having
photopolymerization property by irradiation of activation energy line in a
supercritical fluid or a subcritical fluid.
[WO2007094644]PREPARATION
METHOD OF LITHIUM-METAL COMPOSITE OXIDES/
2007-08-23
Disclosed is a method for preparing a lithium-metal composite oxide, the method
comprising the steps of: (a) mixing an aqueous solution of one or more
transition metal- containing precursor compounds with an alkalifying agent and a
lithium precursor compound to precipitate hydroxides of the transition metals;
(b) mixing the mixture of step (a) with water under supercritical or subcritical
conditions to synthesize a lithium-metal composite oxide, and drying the
lithium-metal composite oxide; and (c) subjecting the dried lithium-metal
composite oxide either to calcination or to granulation and then calcination.
Also disclosed are an electrode comprising the lithium-metal composite oxide,
and an electrochemical device comprising the electrode. In the disclosed
invention, a lithium-metal composite oxide synthesized based on the prior
supercritical hydrothermal synthesis method is subjected either to calcination
or to granulation and then calcination. Thus, unlike the prior dry calcination
method or wet precipitation method, a uniform solid solution can be formed and
the ordering of metals in the composite oxide can be improved. Accordingly, the
lithium- metal composite oxide can show crystal stability and excellent
electrochemical properties.
[EP1816115]Method
for producing glycidiyl ether/
2007-08-08
A method for
producing glycidyl ether using a reaction system in which an organic compound
and water react with each other to generate glycidyl ether and furthermore part
of the reaction product consecutively reacts. A filler for regulating the
consecutive reaction of glycidyl ether is provided in a reaction field of a
fluid mixture of the organic compound and the subcritical water.
[US2007128273]Composition
containing medicine extremely slightly soluble in water and method for
preparation thereof/
2007-06-07
A
composition containing a very low water-soluble drug, which composition is
produced by treating, with a supercritical or subcritical carbon dioxide fluid,
a mixture containing a very low water-soluble drug and a porous material
(exclusive of a porous silica material characterized in that the material has an
average pore diameter of 1 to 20 nm, the total pore volume of the material that
have a diameter falling within a range of +-40% of the average pore diameter
account for 60% or more the volume of all the pores of the material, and, when
subjected to X-ray diffractometry, the material exhibits one or more peaks at a
diffraction angle (2theta) corresponding to d of 1 nm or more); and a method for
producing the composition. The very-low-water-soluble-drug-containing
composition of the present invention ensures improved dissolution of the very
low water-soluble drug.
[WO2007066415]METHOD
OF DYEING FIBROUS MATERIAL OF META-TYPE WHOLLY AROMATIC POLYAMIDE/ 2007-06-14
A fibrous
material of a meta-type wholly aromatic polyamide is dyed in a dyeing system
comprising a subcritical to supercritical carbon dioxide and a dye in the
presence of a polar solvent (e.g., water, methanol, acetone, propanol, or
ethylene glycol). Thus, the fibrous material, which has a rigid molecular
structure and high crystallizability, can be evenly dyed in a high color density
from the surface to the inner part of the fibers.
[JP2007131595]METHOD
FOR PRODUCING FATTY ACID ALKYL ESTER
PROBLEM TO
BE SOLVED: To provide a technology capable of accomplishing yield improvement
etc., of a fatty acid alkyl ester in a method for producing the fatty acid alkyl
ester by using an alcohol solvent.
SOLUTION: The method for producing a fatty acid alkyl ester comprises the first
process wherein a fatty acid glyceride contained in a raw material oil or fat is
hydrolyzed under a supercritical or subcritical condition to yield a fatty acid
and the second process wherein the fatty acid yielded in the first process is
esterified to yield the fatty acid alkyl ester. In the second process, (1) an
alcohol under a supercritical or subcritical condition, (2) a carboxylate under
a supercritical or subcritical condition, and solvents for (1) and (2) are used
together, thus efficiently producing the fatty acid alkyl ester useful as a BDF
(bio-diesel fuel) fuel from the raw material oil or fat
[WO2007046859] EXTRACTION AND
FRACTIONATION OF BIOPOLYMERS AND RESINS FROM PLANT MATERIALS
A method for
the extraction, separation, fractionation and purification of biopolymers from
plant materials using supercritical and/or subcritical solvent extractions is
disclosed. Specifically, the process can be used for the separation of resins
and rubber from guayule shrub (Parthenium argentatum), and other rubber and/or
resin containing plant materials, using supercritical solvent extraction, for
example supercritical carbon dioxide extraction. Additionally, polar and/or
non-polar co-solvents can be used with supercritical carbon dioxide to enhance
the selective extraction of resins and rubbers from the shrub
[RU2295788 ]EXTRACTION
MIXTURE FOR SUPERCRITICAL EXTRACTION OF ACTINIDE OXIDES
ROMANOVSKIJ VALERIJ NIKOLAEVIC (RU); REVENKO JURIJ
ALEKSANDROVICH (RU); KUDRJAVTSEV EVGENIJ GEORGIEVIC (RU); BABAIN VASILIJ
ALEKSANDROVICH (RU); KAMACHEV VLADISLAV ALEKSANDROV (RU); MURZIN ANDREJ ANATOL
EVICH (RU); SHADRIN ANDREJ JUR EVICH (RU); SMIRNOV IGOR VALENTINOVICH (RU); KOMA
JOSHIKATSU (JP); KOJAMO TOMOZO (JP)
[RU2295788]EXTRACTION
MIXTURE FOR SUPERCRITICAL EXTRACTION OF ACTINIDE OXIDES
ROMANOVSKIJ VALERIJ NIKOLAEVIC (RU); REVENKO JURIJ
ALEKSANDROVICH (RU); KUDRJAVTSEV EVGENIJ GEORGIEVIC (RU); BABAIN VASILIJ
ALEKSANDROVICH (RU); KAMACHEV VLADISLAV ALEKSANDROV (RU); MURZIN ANDREJ ANATOL
EVICH (RU); SHADRIN ANDREJ JUR EVICH (RU); SMIRNOV IGOR VALENTINOVICH (RU); KOMA
JOSHIKATSU (JP); KOJAMO TOMOZO (JP)
[JP2007046015]METHOD FOR
PRODUCING CAROTENOID PIGMENT
PROBLEM
TO BE SOLVED: To provide a high safety technique for producing a high quality
plant-derived carotenoid pigment and a high purity and high concentration
[beta]-cryptoxanthin easily and efficiently at a low cost with the use of an
inexpensive and easily available raw material.
SOLUTION: The method for producing a carotenoid pigment comprises a step of
bringing a plant-derived carotenoid pigment-containing substance into contact
with the CO<SB>2</SB>in a critical or subcritical state to an extract carotenoid
pigments, and a specific carotenoid pigment is selectively condensed by
controlling the concentration of an organic solvent in the CO<SB>2

