




|
Inventor(s): [CN]; |
|
Inventor(s): [CN]; |
Abstract of CN105202888 (A)
The invention relates to supercritical fluid drying equipment which comprises a drying body, a filter cavity, a first pipeline and a second pipeline. The drying body comprises a gas drying cavity formed in the upper layer and a heating cavity formed in the lower layer. The bottom layer of the gas drying cavity is obliquely arranged, and a flow channel communicated with the heating cavity is arranged at the bottom of the inclined end. A flow speed induction point, a flow speed control valve and a control valve handle connected with the flow speed control valve are arranged in the flow channel. The control valve handle is arranged on the outer side wall of the drying body. The supercritical fluiddrying equipment comprises the drying body, and is reasonable in structure, good in drying and purifying effect and convenient to maintain.
The invention discloses pre-seasoned peanut oil convenient to cook. The pre-seasoned peanut oil consists of the following raw materials in parts by weight: 950-1000 parts of peanuts, 15-20 parts of onion stalks, 8-10 parts of star aniseed, 6-7 parts of Chinese prickly ash, 4-6 parts of cloves, 6-9 parts of angelica dahurica, 6-8 parts of formosan lattuce herb, 4-5 parts of lalang grass rhizomes, 10-12 parts of sweet-scented osmanthuses, 16-18 parts of abelmoschus esculentus, an appropriate quantity of lactic acid bacteria, and an appropriate amount neutral protease. According to the pre-seasoned peanut oil disclosed by the invention, extraction is performed twice, so that the extraction rate of fat in the peanut oil can be increased; peanut meal is puffed, so that the inner part of the peanut meal and the inner parts of other material compositions form porous structures, and extraction of nutrient substances is facilitated; the lactic acid bacteria and the neutral protease which are added can reduce the content of aflatoxin in the peanut oil, and the content of amino acid in the fat can also be increased; a supercritical carbon dioxide fluid extraction technology is adopted, so that not only can the active component be effectively extracted, but also the production cost can also be reduced; seasonings of the onion stalks, the star aniseed, the Chinese prickly ash and the like are added, so that the process of frequently adding seasonings in the cooking process is omitted. The pre-seasoned peanut oil has the advantages of being convenient and simple, adequate in nutrients, unique in fragrance and the like.
10th International Symposium on
Supercritical Fluids Final Program
ISSF
2012
Welcome
“The Versatility of Supercritical Fluid Technology”
ISSF 2012 is a global conference that brings together an international group of researchers, practitioners, and industrialists who will contribute cutting-edge presentations on emerging applications and processes. The focus of this meeting is to highlight the discovery, development, and production aspects inherent with a wide range of technologies that utilize the unique capabilities of supercritical fluid solvents. This three-day Symposium is structured with parallel sessions packed with plenary, keynote, and contributed talks as well as poster sessions. The official language of the program is English.
Click
below to see the Latest News About ISSF 2012
ISSF 2012 Preliminary Scientific Program - CLICK HERE
ISSF 2012 Brochure - CLICK HERE
NOTE -- the program will end on Wednesday, May 16, at approximately 5:15 p.m.



  |
Monday Poster Session Presentations - I 5:10 to 7:00 pm
Biomass and Energy-Related Conversions
(生物质和能源相关的转换)
Thermodynamics Phase Equilibria-Fluid Properties
(热力学相平衡的流体性质)
Industrial Applications of Critical Fluids
(临界流体的工业应用)Natural Products, Nutraceuticals, and Food-Related Materials
(天然产品,保健品,食品相关材料)Polymers, Materials Applications and Processes
(高分子材料应用和流程)
Reactions in Critical Fluids
(在临界流体的反应)
Tuesday Poster Session Presentations - II 4:45 to 6:30 pm
Pacific Concourse is the location of the Poster Sessions, Exhibits and Breaks
See Author Instructions at http://issf2012.com/author-poster.shtmlMaterials Applications(材料的应用)
CO
2 Remediation and Environmental Aspects (二氧化碳整治和环境方面)Novel SCF Experimental Techniques
(新型超临界试验技术)
Biomedical Applications
(生物医学应用0Green Chemistry, Engineering and Supercritical Fluids
(绿色化学,工程和超临界流体)
Process Design and Economics
(工艺设计和经济学)
Separation Processes
(分离过程)
SCF Particle/Film Technology
(SCF的粒子/薄膜技术)
Pharmaceutical Applications
(制药应用)
Supercritical Fluids-Ionic Liquids/Coupled Media
(超临界流体,离子液体/耦合媒体)
Hydrothermal Processing and Inorganic Materials
(热液处理和无机材料)
Super Green 2004
The
3rd International Symposium on Supercritical
Fluid Technology for Energy and Environment
Applications
( October 23-26, 2004, Tianjin,China)
Background
of Super Green 2004
“Super Green” is an international symposium
covering current applications of supercritical fluid technology in the field of
energy and environment. The purpose of the symposium is to bring together the
scientists and engineers who are working in the field of supercritical fluid
technology in order to share their latest research findings. The idea to have
this kind of symposium was born in 2001, during the summer in the University of
Idaho, USA. Several researchers gathered there and discussed about novel
supercritical fluid technology for energy and environmental applications. The
scientists in this field organized then the 1st and 2nd
International Symposiums on supercritical fluid Technology for energy and
environmental applications separately in Kyung Hee University (Korea) and in
Nagoya University (Japan). The programs have provided maximum exchange of
information on techniques, theory and applications. There were many many
exciting events, many invited lectures by world famous researchers in the
supercritical fluid technology, a short course offered by academic expert as
well as oral and poster presentations. Since both symposiums were very
successful, the International Organizing Committee (IOC) decided to organize the
following symposium of “Super Green 2004” in Tianjin University
of China.
Co-Sponsor:
Tianjin University,China
Institute of Chemical Engineering of China (ICEC)
(Supercritical fluids Technology Society)
Assisted
by :
Institute of Chemistry Chinese Academy of Sciences,China
Supporting
Institutions:
National Natural Science Foundation of China
Ministry of Education P. R. C.
Tianjin Municipal Science and Technology Commission, China
The
Purpose and Scopes of the Symposium
The
purpose of the symposium is to bring together scientists and engineers from
around the world to share their latest research findings on supercritical fluid
technical applications with compressible media (i.e., supercritical,
near-critical, pressurized, solvating gases, high temperature liquids, gas
enhanced liquids) in the fields of energy, environment, medicine and chemical
industries.
|
|
Energy and environment applications based on supercritical fluid technology |
|
|
Nuclear waste and hazardous waste treatments based on supercritical fluid technology |
|
|
Applications of supercritical fluids in materials processing |
|
|
Pharmaceuticals and natural products processing |
|
|
Supercritical fluid chromatography application (analytical and preparative types) |
|
|
Fundamental theoretical research and commercial development of supercritical fluid processing technology |
The symposium will include the following programs:
|
|
Invited lecture presentations featuring leading researchers from industry, government and academia |
|
|
Oral and discussion sessions |
|
|
Poster sessions |
Venture Capita
Name of Project:
10,000T/Y Process and equipment for rectifying tobacco by
supercritical multi-element fluid extraction
This invention is the process
and equipment of tobacco manufacture. Its technical characteristics is adopting
the operating conditions on asset of extraction and rectification devices with
supercritical multi-element fluid such as the temperature and pressure of
variable supercritical master dissolvent,selection and addition of different
varieties of secondary dissolvent and certain addition leval. First to expand
the volume of tobacco by 5~20% ,andthen extract aromatic
absolute,nicotine,tobacco F-1-P crystallin and tobacco SOD erythrocuprein in
rectified tobacco with certain selectivity by steps so as to produce slap-up
essence for tobacco and high vegetal protein beverage.
Feasibility and Necessary Conditions of the Project:
1. Capacity:10,000T/Y.
2. Estimated input: USD 40 million.
3.Utilities: convenient communications and telecommunication, adequate
water, power and gas supply.
Profit
Estimation:
1.Sales income of a year: USD 123.93 million.
2. Annual profit: USD 53.76 million.
3.period of investment: (stable state) 2.3 years (after taxation).
4.Profit rate of investment
5.Intemal financial profit rate: 52.12%,
6.Profit and loss balance point28.05%(2805T/Y).
Brief
Information About Chinese Unelertaker of the Proposed Project:
1. Name: Yunnan Asia-Pacific Zhi-Xin Bio-Engineering Institute.
2. Structure: Joint Stock.
3. Background of the undertaker: The Institute mainly focus on the Indus
on the industrialization of supercritical platonic fluid technology and the
development of production processing automatic control technology. The institute
applied for inventive patent as far as the technology of supercritical multi-element
fluid is concerned. Its patent No. is ZL01104215.X , which has been listed by
the Information Center of China National Science & Tech. Committee, as China
national applied patent and among
key development projects.
4. Construction Site: Kunming High Technological Industrial Development
Zone.
Sources of Foreign Investment:
1. Funds are to be raised
in the following ways:
Stock ( v
) Loan ( v
) Jointly-Funded
( v ) Cooperation
( v )
2.Provision of the
following items and/or agreement to provide fund for them:Equipment ( v )
Permission to enter the international market ( v
)
Fundamental
Facilities Available for the Project:
1.Railway: Guiyang-Kunming, Chengdu-Kunming,
Nanning-Kunming.
2.High-Class Highway:NO.203
National Road.
3.Airport: Kunming International Airport.
4.Harbors:Huangpu, Zhangjiang, Fangchen, Beihai.
Products
and Marker:
|
1.Name of products |
Specification |
Annual output |
Market potential |
|
Nicotine |
98% |
100T/Y |
2,000T/Y |
|
Tobacco absolute |
|
100T/Y |
2,000T/Y |
|
Solanasol |
95% |
100T/Y |
1,000T/Y |
|
CoenzymeQ10 |
98% |
20T/Y |
300T/Y |
|
2.Raw
material |
Annual
Demand |
Unit |
Sources |
|
Tobacco |
11,000 |
Tons |
Tons |
|
CO2 |
300 |
Tons |
Domestic |
|
Ethyl
alcohol |
500 |
Tons |
Domestic |
3
.Sales Orientation: Domestic 30%;
Abroad 70%.
Proposed
Period of Cooperation:
Seeking
for a joint venture partner for 20 years of cooperation.
Inventor
and Patentee:
Mr.
Zhen-kun WANG Add:No.150 Daguan
Road Kunming China. Post
Code:650032
Tel:+86-0871-5332716;013888979128.
E-Mail: sfst@km169.net
Supercritical multi-element fluid Tea Refining (Patent) Project seeking After Partners
|
Application
Number: |
96105251 |
Application
Date: |
1996.05.24 |
|
Publication
Number: |
CN1141727
|
Publication
Date: |
1997.02.05
|
|
Approval
Pub. Date: |
|
|
|
|
International
Classification: |
A23F3/16;
A23F3/30;A23F3/40;A23L1/30;A23K1/16 |
||
|
Applicant(s)
Name: |
WANG
ZHENKUN |
||
|
Inventor(s)
Name: |
WANG
ZHENKUN |
||
Name of
Project:
5500T/Y
Supercritical multi-element fluid
Tea Refining
Introduction:
The
new process of supercritical
multi-element fluid
stepwise
extraction and refining
will be adopted in the project to carry out the further processing of tea.
Compared with the tea processing technology of supercritical CO2
fluid process adopted by two already-operated 6800T/Y tea plants in the world, this new
process can at its extreme raise the comprehensive
utility of tea, increase varieties of products,
improve the quality of products and furthermore can lead to the decrease of the
investment and product cost to a great extent, thus augmenting the product-added
value.
Feasibility and Necessary Conditions of
the Project:
1.
Capacity:5500T/Y.
2. Proposed proportion of capital input: Foreign 55%; Chinese 45%.
3. Estimated input: USD 50 million.
4.Utilities: convenient
communications and telecommunication, adequate water, power and gas supply.
5.According to the
"Regulations Improving Foreign Business in Yunnan Province", from 1
Jan 1997 on, foreign businessmen are allowed to
invest the production of tea caffeine.
Profit
Estimation:
1.Sales income of a year: USD 178.29 million.
2.Total cost of a year: USD 26.82
million.
3.Annual profit: USD 109.34
million.
4.Repayment :422.31%.period of investment: (stable state) 2.3 years (after
taxation).
5.Profit rate of investment
6.Intemal financial profit rate:179.36%.
7.Profit and loss balance point:271.5T/Y(5.43%).
Brief Information About Chinese
Unelertaker of the Proposed Project:
1.
Name: Yunnan Asia-Pacific Zhi-Xin Bio-Engineering Institute.
2. Structure: Joint Stock.
3. Background of the undertaker: The Institute mainly focus on the Indus on the
industrialization of supercritical platonic fluid technology and the development
of production processing
automatic control technology. The institute applied for inventive patent as far
as the technology of supercritical platonic fluid is concerned. Its patent No.
is ZL 96105251.1, which has been listed by the Information Center of China
National Science & Tech. Committee, as China national applied patent and among key development projects.
4. Construction Site: Kunming High Technological Industrial Development Zone.
Sources of Foreign Investment:
1. Funds are to be
raised in the following ways:
Stock ( v ) Loan
( v )
Jointly-Funded ( v )
Cooperation ( v )
2.Provision of the following items
and/or agreement to provide fund for them:Equipment ( v )
Permission to enter the international market ( v
)
Fundamental Facilities Available for
the Project:
1.Railway:
Guiyang-Kunming, Chengdu-Kunming,
Nanning-Kunming.
2.High-Class Highway:NO.203 National Road.
3.Airport: Kunming International
Airport.
4.Harbors:Huangpu, Zhangjiang,
Fangchen, Beihai.
Products
and Marker:
|
1.Name of products |
Specification |
Annual output |
Market potential |
|
Tea
caffeine |
99~101% (USP-22) |
125T/Y |
2,000T/Y |
|
Tea
polyphones |
GTP>95% |
600T/Y |
6,000T/Y |
|
Tea
coloring matter |
|
55T/Y |
1,000T/Y |
|
Tea-Tincture |
>5% |
300T/Y |
3,000T/Y |
|
Other
tea products |
|
3920T/Y |
150,000T/Y |
|
2.Raw material |
Annual Demand |
Unit |
Sources |
|
Teas |
5500 |
Tons |
Tons |
|
CO2 |
300 |
Tons |
Domestic |
|
Ethyl
alcohol |
500 |
Tons |
Domestic |
Proposed Period of
Cooperation:
Seeking
for a joint venture partner for 20 years of cooperation.
Inventor
and Patentee:
Mr. Zheng-kun WANG Add:No.150
Daguan Road Kunming China. Post
Code:650032
Tel:+86-0871-5332716;
E-Mail: sfst@gmw.com.cn
The Product Brief
Introduction
The specific Yunnan
tea products produced by high-tech [supercritical multi-element fluid]
1.
Caffeine
reduced bag tea (The traceable remaining
agrochemicals < international SPS standard)
a.
Pu-erh tea bag (Caffeine: 0.3%)
b.
Yunnan green tea bag (Caffeine: 0.3%)
c.
Yunnan black tea bag (Caffeine: 0.3%)
d.
Oolong tea bag (Caffeine: 0.3%)
e. Pu-erh
composite flower tea bag (Caffeine:
0.3%) The product brief introduction
f.
Jasmine flower tea bag (Caffeine: 0.3%)
2.
The tea polyphenols products
(The traceable remaining metal and organic solvent < international SPS
standard)
a
The tea polyphenols > 50%; Catechins: 25-30%; Caffeine < 1%.
b.
The tea polyphenols > 60%; Catechins: > 30%; Caffeine < 1%.
c.
The tea polyphenols > 70%; Catechins: 40-45%; Caffeine < 1%.
d
The tea polyphenols > 95%; Catechins: >70%; Caffeine < 0.5%.
Herbal perfume for anti-influenza prepared by
Supercritical multi-element fluid extraction technique.
This product is a perfect combination of
topical therapeutics of traditional Chinese Medicine and western aromatherapy.
It is a kind of medicinal cosmetic. It
demonstrates a wonderful activity against influenza and also to be an
environmently-friendly perfume.
Influenza is widespread disease with a high
infection rate. More than 90% of the population is infected every year. To
control and treat is a challenge
even for modern western medicine. However, Traditional Chinese Medicine
especially the medicinal perfume condensed bioactive essential components showed
a definite advantage in this aspect.
Our product (or the name of product) can be
taken through the respiratory system and has proven activity against influenza.
It has no side effects.
SOD product
from edible plants
SOD is a very important free radical scavenger.
It shows various bioactivities such as anti-inflammatory, antitumor, anti-aging,
internal detoxification and ultraviolet free radical scavenging. It also
enhances the self-immunology ability. It has seen widespread use in the medical,
health food and medicinal cosmetics industries.
SOD isolated from the plants is the best
replacement to those obtained from beef or pork.
We provide the following SOD products with a
competitive price and guaranteed quality.
1.
SOD lyophilized powder: Activity > 20000 U/g; 100 kg product is
currently available and 800 kg product is under preparation.
2.
The wine fermented from the wild fruits from the mountains of Yunnan.
The SOD quantity:
>600 U/ml in a bottle of 500 ml.
3.
The capsule of the flower powder from the eatable flower in Yunnan
mountains.
The SOD quantity:
>200 U/per capsule.
Supercritical technique is abroad latest
trend(2005)
1)Microemulsions in supercritical CO2 utilizing the polyethyleneglycol
dialkylglycerol and their use for the solubilization of hydrophiles
Dyes and Pigments, Volume 65,
Issue 1, April 2005, Pages 67-74
Abstract
Novel
polyethyleneglycol dialkylglycerol surfactants, 3-methyl-penta
ethyleneglycol-2-octyl-1-pentylglycerol
(CH3(OCH2CH2)5OCH2CHO((CH2)7CH3)CH2O(CH2)4CH3),
3-methylpentaethyleneglycol-2-pentyl-1-octylglycerol (CH3(OCH2CH2)5O
CH2CHO((CH2)4CH3)CH2O(CH2)7CH3),
3-methylpentaethyleneglycol-2-(2-ethylhexyl)-1-octhylglycerol
(CH3(OCH2CH2)5OCH2CHO(CH2CH(C2H5)(CH2)3CH3)CH2O(CH2)7CH3),
3-methylpentaethyleneglycol-2-(2-ethylhexyl)-1-(2-ethylhexyl)glycerol
(CH3(OCH2CH2)5CH2CHO(CH2CH(C2H5)(CH2)3CH3)CH2OCH2CH(C2H5)(CH2)3CH3), were
synthesized to evaluate the solubility and the possibility for the formation of
stable microemulsions in supercritical CO2. The surfactants synthesized in this
study were satisfactorily dissolved in supercritical CO2. However, the quantity
of water, which can dissolve into the same system, was very little. It has also
become obvious that small water droplets in CO2 microemulsions stabilized by
synthesized surfactants have an ability to incorporate hydrophiles,
water-soluble dyes, at the same system.
2)Supercritical fluids: technology and application
to food processing
Journal of Food Engineering, Volume 67, Issues 1-2, March 2005, Pages 21-33
Abstract
Supercritical fluids (SCFs) are substances at pressures and temperatures above
their critical values. It is characteristic that properties of SCFs can be
changed in a wide range. Their solvent power is the highest for non-polar or
slightly polar components and decreases with increasing molecular weight. They
can easily be removed from the solutes by mere expansion to ambient pressure.
Carbon dioxide (CO2) is particularly advantageous for processing food materials.
SCFs are used for batch extractions of solids, for multi-stage counter-current
separation (fractionation) of liquids, and for adsorptive and chromatographic
separations. State of the art design for commercial plants is available, and a
number of installed plants are working. Special applications to food processing
include decaffeination of green coffee beans, production of hops extracts,
recovery of aromas and flavours from herbs and spices, extraction and
fractionation of edible oils, and removal of contaminants, among others. The
application of SCFs is now extended to new areas like formulation or specific
chemical reactions. Costs of SCF extraction (SCFE) processes are competitive. In
certain cases SCFE processing is the only way to meet product specifications.
3)Contributions to supercritical extraction of vegetable substrates in
Latin America
Journal of Food Engineering, Volume 67, Issues 1-2, March 2005, Pages 35-57
Abstract
This
manuscript summarizes basic and applied research on phase equilibrium and mass
transfer kinetics involved in high-pressure CO2 extraction of solid substrates.
Most examples relate to the extraction of lipids and essential oils from native
Latin American plants. Extraction rates of vegetable matrices depend on the
external mass transfer coefficient (kf), effective solute diffusivity in the
solid substrate (De), solute solubility in high-pressure CO2, and solute binding
to the solid matrix. The initial stages of the extraction process depend on an
operational solubility that is close to the thermodynamic solubility (csat) in
the case of lipid extraction from oil-containing plant material, but lower than
csat in the case of essential oils, due probably to stronger interactions
between essential oils than lipids and the solid matrix. Experimental values of
kf exhibited considerable scattering and were several orders of magnitude
smaller than corresponding values from literature correlations for the
dissolution of solids or evaporation of liquids from films with supercritical
fluids (SCFs), due to underestimation of the contribution of internal (solid
phase) mechanisms to the total resistance to mass transfer and other aspects. De
values were 10–103 or 102–105 times smaller than binary diffusion coefficients of
lipids and essential oils, respectively, in high-pressure CO2, suggesting very
pronounced limitations to mass transfer within the solid matrices in both cases.
The integration of this information for the modeling, simulation, and scaling-up
of laboratory data is thoroughly discussed. Finally, an example of economic
feasibility is given for the installation of a SCF extraction plant for the
recovery of lipids from wheat germ.
4)Rapid estimation of the manufacturing cost of
extracts obtained by supercritical fluid extraction
Journal of Food Engineering, Volume 67, Issues 1-2, March 2005, Pages 235-240
Abstract
In spite of the scientific knowledge and the large availability of raw materials
having sufficient quality and cost, there is no industrial supercritical fluid
extraction unit in any of the South American countries. Supercritical fluid
extraction is associated with high investment costs; nowadays, an easy method
for technical–economical
evaluation of supercritical fluid process is not available. Thus, a simple
method to estimate the cost of manufacturing of extracts by supercritical fluid
technology is presented. The manufacturing costs of clove bud oil and ginger
oleoresin were estimated using the procedure proposed. The production of clove
bud oil was economically feasible at the quoted extraction condition; its
manufacturing cost approximately a fourth of the market price. The manufacturing
cost of ginger oleoresin was close to its selling price at the extraction
condition considered. This is mainly due to the strong influence of the
investment on the cost of manufacturing ginger extracts by supercritical
extraction due to the requirement of long extraction times. Nonetheless, some
other characteristics of the ginger oleoresin such as the quantity and the
availability of gingerols and shogaols should be considered. Additionally,
further process parameter studies directed to the increase of the extraction
rates should be considered before disregarding the supercritical fluid
extraction as a viable process.
5)Microencapsulation of particles using
supercritical carbon dioxide
Chemical Engineering and Processing, Volume 44, Issue 2, February 2005, Pages
215-219
Abstract
In this contribution a novel fluidized-bed coating process is introduced to
encapsulate heat-sensitive materials with particle sizes below 100 m.
Supercritical carbon dioxide is used as solvent for the coating material as well
as carrier fluid for the core material. The behaviour of the high pressure
fluidized-bed was investigated for different process parameters and materials.
It is shown that the fluidization starts at lower fluid velocities if the
pressure is increased and it was possible to fluidized particles with a mean
size below 10 m. The coating of glass beads with stearyl alcohol was carried out
and layers with a thickness of 1–8
m were achieved.
6) Separation of parthenolide from feverfew: performance of conventional
and high-pressure extraction techniques
Separation and Purification Technology, Volume 41, Issue 1, January 2005, Pages
13-20
Abstract
In
present work the extraction of feverfew flower heads was performed using
supercritical carbon dioxide at pressures from 200 to 800 bar and at
temperatures of 40, 60 and 80 °C. For comparison, the conventional extractions
with organic solvents have been performed. Dry feverfew flower heads were used
as starting material. Supercritical fluid extraction (SFE) was performed using a
semi continuous flow apparatus in a laboratory and pilot scale. The influence of
process parameters on the total yield and amount of parthenolide isolated was
investigated. Dynamic behaviour of the extraction runs followed by single-step
separation was analysed by a mathematical model for initial constant rate
extraction period and the subsequent time-dependant diffusion controlling mass
transfer rate period. In order to concentrate the obtained extract in
parthenolide, a two-step separation was employed.
7)Mathematical model for supercritical fluid
extraction of natural products and extraction curve evaluation
The Journal of Supercritical Fluids, Volume 33, Issue 1, January 2005, Pages
35-52
Abstract
New model for supercritical fluid extraction (SFE) of natural products is
presented. Like other models based on the concept of broken and intact cells, it
is particularly suited to fit experimental data as it almost independently
simulates two extraction periods, the first one governed by phase equilibrium
and the second one governed by internal diffusion in particles. Its new feature
is a detailed description of the first extraction period where different types
of phase equilibrium and solvent flow patterns are taken into account. A
simplified approximate form of the model is used to analyse its properties and
to estimate its parameters. The number of model parameters is, in dependence on
the complexity of the extraction process, 1–3 for phase equilibrium, 2–3 for mass transfer and 1 for flow pattern. The model is
versatile, but, as a consequence, more data are necessary than a single
extraction curve to determine its parameters in the first period. The evaluation
of model parameters from extraction curves is shown on data sets from
literature.
8)Essential oil composition and antimicrobial
activity of Origanum majorana L. extracts obtained with ethyl alcohol and
supercritical carbon dioxide
Food Research International, Volume 38, Issue 1, January 2005, Pages 51-57
Abstract
Volatile
components of marjoram (Origanum majorana L.) essential oil obtained by
hydrodistillation and extracts obtained by solvent extraction with ethyl alcohol
and supercritical fluid extraction (SFE) were investigated. The compositions of
volatile compounds in essential oil, ethanolic and SFE extracts were determined
by GC and GC–MS.
The antimicrobial properties of marjoram solvent extracts were investigated with
microbiological tests against food borne fungi and bacteria strains. Extracts
obtained by SFE at high pressure and temperature showed significantly stronger
antimicrobial properties in comparison to the slight inhibitory effects of the
ethanolic extract. The results support the notion that extracts obtained by SFE
might have a role as flavourings and natural colourants as well as use as
preservatives in food and cosmetic systems.
9)Supercritical carbon dioxide extraction of
tagitinin C from Tithonia diversifolia
The Journal of Supercritical Fluids, Volume 33, Issue 1, January 2005, Pages
53-59
Abstract
Different parameters as temperature, pressure, solvent mass and sample
granulometry governing the extraction yield of tagitinin C from the aerial parts
of Tithonia diversifolia were optimised.
An experimental design was carried out to map the effects of pressure (at 20.3,
30.4 and 40.5 MPa) and temperature (at 40, 60 and 80 °C) on the extraction
yield of the active component and to determine the optimal conditions for the
extraction of tagitinin C from T. diversifolia. The best conditions are met for
a pressure of 35.0 MPa and a temperature of 68 °C.
The effect of the particle size was studied under low pressure (13.7 MPa) and
temperature (40 °C) conditions, which failed to extract quantitatively the
tagitinin C from leaves sieved to 250 m size. The reduction of the particle size
increased the extraction yield which became comparable to that of the optimised
SFE for the particle in the range of 0–63 m.
From
the analysis of extraction kinetic curves of 200 mg of plant with supercritical
carbon dioxide (range of 5–30 g), it appears that 15 g of this supercritical fluid is
never limiting.
The
optimised supercritical fluid extraction (SFE) was compared favourably to
Soxhlet extraction with dichloromethane (S) and to maceration followed by
lixiviation with diethyl ether (ML), which gave similar extraction yields but
higher extract content of tagitinin C were found using SFE (15.6 and 30.7% w/w
tagitinin C in S and ML extracts, respectively, versus 52.8% in SFE extract).
10)Supercritical fluid extraction and
high-performance liquid chromatography–fluorescence
detection method for polycyclic aromatic hydrocarbons investigation in vegetable
oil
Food Control, Volume 16, Issue 1, January 2005, Pages 59-64
Abstract
In
spite of the fact that food processes, that involve drying and smoking, may
cause polycyclic aromatic hydrocarbon contamination, an extraction clean/up
procedure carried out by SFE was developed in order to isolate polycyclic
aromatic hydrocarbons from oil vegetable samples for subsequent HPLC–FL
determination. The detection and quantification limits obtained were <1.55 g
kg−1 oil and <2.55 g kg−1 oil, respectively, allowed to check the
presence of seven of the eight PAHs with legal limit in olive–pomace oil: benzo[a]anthracene, benzo[e]pyrene,
benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene,
dibenzo[ah]anthracene and benzo[ghi]perilene. In brief, the method permits the
evaluation of edible oil safety and, therefore, consumers protection.
11)Supercritical fluid extraction of carotenoids
and chlorophyll a from Nannochloropsis gaditana
Journal of Food Engineering, Volume 66, Issue 2, January 2005, Pages 245-251
Abstract
Traditional
methods for the extraction of carotenoids and chlorophylls from microalgae
frequently require more than one extraction step with organic solvents, which
are forbidden in the processing of food additives. In addition, further process
steps are necessary for the separation of carotenoids from chlorophylls.
Consequently, faster processing methods that are compatible with food production
are extremely important.
The aim of this study was to ascertain the influence of pressure and temperature
on the supercritical fluid extraction of carotene and chlorophyll from a
freeze-dried powder of the marine microalgae Nannochloropsis gaditana. The
operating conditions were as follows: pressures of 100, 200, 300, 400 and 500
bar and temperatures of 40, 50 and 60 °C. The extracts were analysed by
measuring the absorbance at 665 and 480 nm. Empirical correlations were also
developed.
The results demonstrate that it is necessary to work at a pressure of 400 bar
and a temperature of 60 °C to obtain a significant yield in the extraction of
the pigments. The best Carot/Chlor ratio was obtained at 200 bar and 60 °C. It
was also found that excellent selectivity can be obtained under these operating
conditions and this could enable the separation and purification of these kinds
of extracted pigments.
12) A new correlation of solubilities of azoic
compounds and anthraquinone derivatives in supercritical carbon dioxide
The Journal of Supercritical Fluids, Volume 32, Issues 1-3, December 2004, Pages
27-35
Abstract
Solubilities of azoic compounds and anthraquinone derivatives in supercritical
carbon dioxide were correlated with a new semiempirical equation and five
equations taken from literature. All solutes are dyestuffs or compounds with a
molecular structure similar to dyestuffs. The equations were applied to
literature solubility data [J. Supercrit. Fluids 21 (2001) 1; J. Supercrit.
Fluids 13 (1998) 37; Fluid Phase Equilib. 158–160 (1999) 707; J. Supercrit. Fluids 13 (1998) 43; Talanta
48 (1999) 951; Fluid Phase Equilib. 200 (2002) 31; Fluid Phase Equilib. 194–197
(2002) 895; J. Supercrit. Fluids, in press] and to a new data set of solvent
brown 1, experimentally measured by us. Altogether, 16 compounds and over 400
solubility data were considered. In the development of the new correlation,
which has three fitting parameters, a dimensionless group was defined: this
group, which involves solute molar volume and fusion properties, is a function
of solvent density over a significant range of pressures and temperatures. The
proposed equation is able to correlate solubility data with good agreement. The
mean average absolute percent deviation (AA%D) of the new equation was the
lowest among the equations with the same number of fitting parameters.
Supercritical technique is abroad latest
trend(2004)
A method for dissociating metal-ligand complexes in a supercritical
fluid by treating the metal-ligand complex with heat and/or reducing or
oxidizing agents. Once the metal-ligand complex is dissociated, the resulting
metal and/or metal oxide form fine particles of substantially uniform size. In
preferred embodiments, the solvent is supercritical carbon dioxide and the
ligand is a .beta.-diketone such as hexafluoroacetylacetone or dibutyldiacetate.
In other preferred embodiments, the metals in the metalligand complex are
copper, silver, gold, tungsten, titanium, tantalum, tin, or mixtures thereof. In
preferred embodiments, the reducing agent is hydrogen. The method provides an
efficient process for dissociating metal-ligand complexes and produces
easily-collected metal particles free from hydrocarbon solvent impurities. The
ligand and the supercritical fluid can be regenerated to provide an economic,
efficient process.
ULTRASONICALLY
ENHANCED PROCESS FOR EXTRACTION OF METAL SPECIES IN SUPERCRITICAL FLUID
PROBLEM TO
BE SOLVED: To efficiently dissolve or extract metals, such as uranium,
platinum and palladium, metalloids or their oxides while reducing environmental
load.
SOLUTION: The metals, metalloids or their oxides are dissolved or extracted by
using a supercritical fluid, such as carbon dioxide, containing a chelating
reagent such as alkyl phosphate and a Lewis acid such as mineral acid and
applying ultrasonic vibrations at about 300 to 350 K under about 10 to 25 MPa
pressure.
FLUID
EXTRACTION OF METALS OR METALLOIDS
Methods for extracting metalloid and metal species from a solid or
liquid material by exposing the material to a fluid solvent, particularly
supercritical CO2, and a chelating agent is described. The chelating agent forms
a chelate with the species, the chelate being soluble in the fluid to allow
removal of the species from the material. In preferred embodiments the
extraction solvent is supercritical CO2 and the chelating agent comprises an
organophosphorous chelating agent, particularly sulfur-containing
organophosphorous chelating agents, including mixtures of chelating agents.
Examples of chelating agents include monothiophosphinic acid, dithiophosphinic
acid, phosphine sulfite, phosphorothioic acid, and mixtures thereof. The method
provides an environmentally benign process for removing metal and metalloids
from industrial waste solutions, particularly acidic solutions. Both the chelate
and the supercritical fluid can be regenerated and the contaminant species
recovered to provide an economic, efficient process.
Determining phase boundaries and vapour/liquid critical points in
supercritical fluids: a multi-technique approach
We describe an apparatus used to make precise determinations of the coexistence
regimes and critical points of single, binary, and multi-component fluids. The
design allows semi-automated measurements to be made over a pressure range of
1–300 bar and a temperature range of−20 to +120 °C with a
stability of ±2 mK. We describe the use of the apparatus in determining
the critical points and phase boundaries of single component and binary mixtures
through a number of optical, acoustic, and shear-mode piezoelectric sensor
methods. We conclude that the accurate determination of bubble-point lines,
dew-point lines and critical points is best achieved by making measurement using
a number of complementary techniques.
Solubility of N-CBZ derivatised amino acids in supercritical carbon
dioxide
Slaughterhouse/tannery wastes such as fleshings, hair which are made up of
proteins are not utilized properly even though they are rich in amino acids.
Proteins are hydrolysed to amino acids and separation of amino acids involves
several process steps. In most of the processes reported, all the amino acids
were not totally separated. Supercritical solvents are having special
properties, which are absent in conventional liquid based solvents however, they
are not suitable for polar substances. Amino acids solubility in supercritical
carbon dioxide is very low, hence a study has been made to measure solubility of
modified amino acids (N-CBZ amino acids). This modification showed
increased solubility. By suitable derivatisation and by means of blocking both
the polar groups (COOH, NH2) the solubility of these amino acids can
be increased further and supercritical fluid extraction technique can be used
for separation of amino acids.
An experimental technique for measuring high solubilities of dyes in
supercritical carbon dioxide
The solubilities of colour index (C.I.) disperse orange 3, red 324, blue 79
and quinizarin were measured at the temperatures of 353.2, 373.2, 393.2 K
between 18 and 30 MPa with a ‘flow method’ conceived for determination of
high solubilities in order to avoid the flow instability problems connected with
dye precipitation. The comparison of results with the literature underlines the
advantage of this new technique: higher solubility values are obtained
especially at the most severe operating conditions. Experimental data for
disperse orange 3 were also compared with similar results obtained by the
authors using a ‘batch method’ in a previous work. The comparison of the
results shows how the ‘flow method’, even though adapted for high solubility
measurements, reveals to be the simplest and more efficient technique to
evaluate dye solubility in supercritical fluids.
Innovative supercritical CO2 extraction of lycopene from tomato in
the presence of vegetable oil as co-solvent
This work describes an innovative process for the
extraction of lycopene from tomato using supercritical carbon dioxide in the
presence of vegetable oil as co-solvent. The presence of the co-solvent improves
the yields of the lycopene extract and has a beneficial role in the stability of
the pigment. A complete description of the extraction process is also reported.
The experiments carried out with and without co-solvent at pressures and
temperatures ranging from 335 to 450 bar and 45 to 70 °C, respectively,
bar have shown that the amount of the extractable lycopene depends on the
experimental conditions. Also, the maximum amount of the extractable lycopene
from dried tomato (6% of moisture, average particle size of about 1 mm), at 450
bar and 66 °C in the presence of co-solvent and utilizing a flow rate of
about 20 kg CO2/h, was 60%. The extracts were analyzed by
high-performance liquid chromatography and UV–vis spectra.
Supercritical antisolvent fractionation of propolis tincture
Propolis is used by bees for strengthening and waterproofing a hive, and for
sterilizing the hive against microbial infections. Propolis contains a high
concentration of flavonoids, which are used in a wide range of cosmetic and
health food preparations for their antimicrobial properties. Propolis is usually
dissolved in ethanol or ethanol/water mixtures to remove insoluble material such
as waxes and detritus from the hive. The resultant solution is a propolis
tincture. A new supercritical antisolvent/extraction process has been developed
for the fractionation of propolis tincture to obtain flavonoids and essential
oil fractions by extraction, and remove high molecular mass components by
antisolvent precipitation. Flavonoids are practically insoluble in pure CO2,
but sufficiently soluble in CO2+ethanol to enable their separation
from high molecular mass and/or more polar components. In the first step of the
process, supercritical CO2 is used both as an anti-solvent to
precipitate high molecular mass components, and as a solvent to extract the
ethanol and soluble components of the propolis. This extract is then
fractionated in two separation steps to create a concentrated flavonoid fraction
as the primary product, and an essential oil/ethanol fraction as a secondary
product. The effects of pressure, temperature, flow rate ratio, tincture
composition and tincture concentration on product quality and yield were
determined at a laboratory and pilot scale. The tincture concentration of
propolis has the greatest effect on the yield and concentration of flavonoids in
the product fraction when pure ethanol is used as the solvent. The flow rate
ratio becomes important when the tincture also contains water. The process has
been successfully scaled up to a demonstration scale using optimized pressure,
temperature, flow ratio and tincture concentrations obtained from laboratory and
pilot scale trials.
Supercritical
technique is abroad latest trend(2004)
Corrosion
in high-temperature and supercritical water and aqueous solutions: a review
The aim of the present article is to review some
of the common corrosion phenomena and describe the predominant corrosion
mechanisms in high-temperature and supercritical water. Corrosion in aqueous
systems up to supercritical temperatures is determined by several
solution-dependent and material-dependent factors. Solution-depending factors
are the density, the temperature, the pH value, and the electrochemical
potential of the solution, and the aggressiveness of the attacking anions.
Material-dependent parameters include alloy composition, surface condition,
material purity, and heat treatment. Corrosion phenomena that are observed
include intergranular corrosion, pitting, general corrosion, and stress
corrosion cracking. The solubility and dissociation of both attacking species
and corrosion products play the most important role for corrosion in
high-temperature water. Both solubility and dissociation processes are strongly
influenced by the density, or the ionic product, respectively, of the solvent.
High values of both parameters favor ionic reactions, and thus, accelerate
electrochemical forms of corrosion. At low densities, water behaves like a
non-polar solvent, and thus, ions associate. In these cases, the concentation of
e.g. aggressive H+ drops down and thus, solutions containing species
such as HCl become neutral and thus less aggressive. Further, corrosion products
plug the surface and material loss stops. Materials parameters have influence
especially on the initiation of corrosion. In the present article, these factors
are linked with the physical and chemical properties of high-temperature and
supercritical water. An outlook is also given for future research needs.
Water
gas shift reaction kinetics under noncatalytic conditions in supercritical water
The kinetics of the water gas shift reaction was
studied under noncatalytic conditions in supercritical water at CO/H2O
ratios of 0.03 and at temperatures from 653 to 713 K. The selectivities of CO2
and hydrogen were almost equal and did not change with pressure at 673 K. The
increase of pressure and water density sensitively promoted the reaction at 653
K from 25 to 30 MPa whereas the pressure and water density did not seem to
affect the rate constant at 673 K from 10 to 30 MPa. The first order rate
constant for CO conversion was k=105.58±1.38 exp(-1.16±0.19×105/RT)
/s at 10–59.6 MPa and 653–866 K. Water density dependence of formaldehyde
reaction in supercritical water
The water density dependence of formaldehyde (HCHO)
reaction in supercritical water (SCW) was studied with batch experiments. Major
products from the reaction were methanol (CH3OH), formic acid (HCOOH),
hydrogen (H2), carbon monoxide (CO) and carbon dioxide (CO2).
It was found that the Cannizzaro reaction mechanism was the preferred reaction
pathway for HCHO reaction in SCW. At higher water densities, CH3OH
yields increased confirming the predominance of the Cannizzaro reaction
mechanism. At low water densities, CO yields increased and CH3OH
yields decreased, which indicated that monomolecular decomposition became the
main reaction pathway. Addition of base to the reacting mixtures was found to
promote the Cannizzaro reaction path whereas addition of acid promoted
monomolecular decomposition.
A
double-wall reactor for hydrothermal oxidation with supercritical water flow
across the inner porous tube
Supercritical water oxidation (P>22.1
MPa, T>647 K) is an efficient process to treat hazardous organic
compounds: high destruction rates (>99.9%) with no NOx production,
rapidity and a good confining of the reaction. Its performances are limited by
salt precipitation and corrosion. A new reactor has then been developed to solve
these problems. It consists in a concentric double wall reactor in which the
corrosive reactants are maintained inside an alumina porous tube whereas
pressure resistance is ensured by a stainless steel external vessel. A water
flow through the porous pipe prevents sticky solid particles from depositing on
the wall. The performances of this reactor were investigated. At 723 K and 25
MPa, the destruction of methanol, used as a model compound, reached 99.9%. Due
to high thermal gradients generated by the exothermic reaction, the pipe which
plays an important role in the decrease of salt precipitation and corrosion can
be broken. Thus, its behaviour must be controlled in-situ. The pressure drop
measurement across the porous wall was used to check whether the inner pipe was
still intact. In fact, the experimental results show that supercritical water
flow through the tube follows the Darcy's law. The experiments also confirm that
the porous medium permeability is a characteristic constant of this medium. The
permeability value remains equal whichever the fluid used, liquid water or
supercritical water. In addition, the pressure drop measurement across the
porous wall allows the control of the tube integrity.
Anti-solvent
and co-solvent effect of CO2 on the solubility of griseofulvin in
acetone and ethanol solutions
A
synthetic method was used for measuring the solubility of griseofulvin in
acetone–CO2 and ethanol–CO2 mixtures. In this method,
CO2 is added gradually to a liquid solution previously introduced in
a sapphire cell of variable volume. Resulting mixtures may have compositions
richer in organic solvents than in CO2, close to compositions found
in the batch anti-solvent process. Measurements with the griseofulvin–CO2–acetone
system were made at 312.15 K at 60 and 100 bar, and at 326.15 K and 100 bar.
Concerning the griseofulvin–CO2–ethanol system, investigations
were carried out at 100 bar at temperatures of 312.15 and 326.15 K. The
solubility of griseofulvin in acetone decreases at all investigated conditions
when the CO2 is added. In this case, CO2 is acting as an
anti-solvent. In contrast to this, griseofulvin solubility in ethanol–CO2
mixtures is higher than the solubility in either pure solvents within a certain
range of CO2 molar fractions. In this case, CO2 is acting
as a co-solvent and promotes the solubility of griseofulvin.
Innovative
supercritical CO2 extraction of lycopene from tomato in the presence
of vegetable oil as co-solvent
This
work describes an innovative process for the extraction of lycopene from tomato
using supercritical carbon dioxide in the presence of vegetable oil as
co-solvent. The presence of the co-solvent improves the yields of the lycopene
extract and has a beneficial role in the stability of the pigment. A complete
description of the extraction process is also reported. The experiments carried
out with and without co-solvent at pressures and temperatures ranging from 335
to 450 bar and 45 to 70 °C, respectively, bar have shown that the amount
of the extractable lycopene depends on the experimental conditions. Also, the
maximum amount of the extractable lycopene from dried tomato (6% of moisture,
average particle size of about 1 mm), at 450 bar and 66 °C in the presence
of co-solvent and utilizing a flow rate of about 20 kg CO2/h, was
60%. The extracts were analyzed by high-performance liquid chromatography and
UV–vis spectra.
Supercritical
and near-critical CO2 in green chemical synthesis and processing
Carbon
dioxide is often promoted as a sustainable solvent, as CO2 is
non-flammable, exhibits a relatively low toxicity and is naturally abundant.
However, injudicious use of carbon dioxide in a process or product can reduce
rather than enhance overall sustainability. This review specifically examines
the use of CO2 to create greener processes and products, with a focus
on research and commercialization efforts performed since 1995. The literature
reveals that use of CO2 has permeated almost all facets of the
chemical industry and that careful application of CO2 technology can
result in products (and processes) that are cleaner, less expensive and of
higher quality. Solubility of coenzyme Q10 in supercritical carbon dioxide
The
equilibrium solubility of coenzyme Q10 (CoQ10) in supercritical carbon dioxide
(scCO2) was measured by a static analytical method in the pressure
range from 9 to 26 MPa, at temperatures of 305, 313 and 323 K. The cosolvent
effect of ethanol in the solubility of the bioactive compound in scCO2
has been investigated, at 15 MPa and 313 K. A preliminary study of the viability
of extracting CoQ10 with scCO2 has been investigated at 15 MPa and
313 K, using the content of commercial pharmaceutical capsules as the solid
matrix feed. The solubility data results were correlated by use of the empirical
density-based Chrastil model.
Solubility
behavior of ethyl cellulose in supercritical fluid solvents
Solubility
data to 180 °C and 1200 bar are reported for ~1.0 wt.% ethyl cellulose
(50% ethoxyl content, 2.5 average degree of substitution) (EC) in neat
supercritical fluid (SCF) chlorodifluoromethane (F22); difluoromethane;
1-chloro-1,1-difluoroethane; 1,1-difluoroethane; and dimethyl ether (DME). The
pressures needed to dissolve EC in the polar fluorocarbons decreases with
increasing solvent size. The exception in this trend is F22 which is the best
fluorocarbon solvent of the series likely due to its ability to hydrogen bond to
the oxygens in EC. Data are also reported for EC in CO2 with up to 30
wt.% ethanol and methanol showing that, on a weight basis, methanol is a much
better cosolvent although on a mole basis methanol is only slightly better. DME
is the highest quality solvent for EC of the series of SCF solvents
investigated. Although the EC+DME system exhibits lower critical solution
temperature behavior similar to the EC+F22 system, EC dissolves in DME at lower
temperatures and pressures compared with F22. Solution density data at the phase
boundaries are reported for the EC+SCF solutions. The EC+DME solutions exhibit
the lowest densities which suggests that EC-DME cross interactions are very
strong and likely dominated by hydrogen bonding.
A
semicontinuous flow apparatus for measuring the solubility of opaque solids in
supercritical solutions
A
semi-continuous flow apparatus with microsampling has been developed for
measuring the solubilities of solids in supercritical fluids. The apparatus is
particularly appropriate for those solids for which only mg-sized quantities are
available (e.g. pharmaceuticals) and which form opaque solutions when dissolved
in supercritical solvents (e.g. porphyrins and dyes). The method of operation is
to first obtain equilibrium in a variable-volume view cell, then use an
electronic syringe pump in the constant pressure mode to deliver the contents of
the cell through a sample loop at the equilibrium temperature and pressure, and
finally to determine solid solubilities by analyzing the contents of the sample
loop. Both an error analysis and measurements for the system carbon dioxide (CO2)+phenanthrene
at 35 and 55 °C indicate that the method is accurate to better than ±5%.
Subsequently, the apparatus was used to determine the solubilities of
5,10,15,20-tetrakis(3,5-bis(trifluoromethyl)phenyl)porphyrin (TBTPP), a novel
fluorinated porphyrin synthesized in our laboratories, in supercritical CO2.
Measurements were performed at three different temperatures (40, 70, and 100 °C)
and over a pressure range of 103.4–324.1 bar. TBTPP exhibits a wide range of
solubilities in CO2 (i.e. from 0.0002 to 2 wt.%) and thus is an
appropriate model solute for investigating the effect of concentration on
particle size and morphology during rapid expansion processing.
Modeling
of extraction of
![]() -carotene
from apricot bagasse using supercritical CO2 in packed bed extractor
-carotene
from apricot bagasse using supercritical CO2 in packed bed extractor
This
work investigates the modeling of
![]() -carotene
extraction from industrial waste product of apricot bagasse at the production of
fruit juice. Shrinking core model was selected as the best mathematical model,
which characterize the extraction process, after take into consideration of mass
transfer mechanisms such as adsorption, diffusion, solubility, and desorption.
Effect of main separation parameters such as pressure, temperature, CO2
flow rate, and particle size on the extraction yields were researched at the
supercritical fluid extraction system of laboratory scale and the results were
compared with the results obtained from the solution of mathematical model.
-carotene
extraction from industrial waste product of apricot bagasse at the production of
fruit juice. Shrinking core model was selected as the best mathematical model,
which characterize the extraction process, after take into consideration of mass
transfer mechanisms such as adsorption, diffusion, solubility, and desorption.
Effect of main separation parameters such as pressure, temperature, CO2
flow rate, and particle size on the extraction yields were researched at the
supercritical fluid extraction system of laboratory scale and the results were
compared with the results obtained from the solution of mathematical model.
The Journal of Supercritical Fluids[Popular thesis in 2003]
1)、Reactions
of supercritical alcohols with unsaturated hydrocarbons
The reactions of some supercritical alcohols were
investigated using 1,1-diphenylethylene, styrene, allylbenzene, and
diphenylacetylene as the reaction partners. 1,1-Diphenylethylene in
supercritical methanol was hydroxymethylated to afford 3,3-diphenyl-1-propanol
as the major product. The alkenes containing a single and no conjugate phenyl
group were also hydroxymethylated in supercritical methanol, but the reaction
rates were significantly reduced when compared with that for
1,1-diphenylethylene. Styrene was converted to the hydroxyalkylated products in
supercritical ethanol and 2-propanol as well as in supercritical methanol. The
rates of the hydroxyalkylation of styrene were strongly dependent on the
structures of the supercritical alcohols; the order of reactivity was
2-propanol>ethanol>methanol. The relation between the structures of the
alcohols and the rates of hydroxyalkylation suggests that the reaction begins
with an attack on the electrophile (+CR2OH or ![]() +CR2OH)
by the pi electrons of the styrene double bond. All the examined alkenes
afforded their hydrogenated derivatives other than the hydroxyalkylated ones. In
addition, supercritical alcohols acted as hydroxyalkylating or hydrogenating
reagents for the triple bond in the reaction with diphenylacetylene.
+CR2OH)
by the pi electrons of the styrene double bond. All the examined alkenes
afforded their hydrogenated derivatives other than the hydroxyalkylated ones. In
addition, supercritical alcohols acted as hydroxyalkylating or hydrogenating
reagents for the triple bond in the reaction with diphenylacetylene.
2)、Stainless
steel flow reactor for supercritical water oxidation: corrosion tests
One of the obstacles that is inhibiting the
development of supercritical water oxidation (SCWO) into a viable industrial
process, is the problem of corrosion. A bench scale stainless steel flow reactor
for supercritical water oxidation studies was constructed. Corrosion of the
reactor was studied under pressure of 400 bars and at temperatures of 250, 375
and 420 °C. The concentrations of various metals in the effluent were
monitored by flame atomic absorption spectrometry. Higher corrosion rates were
observed at 375 °C, or near the critical temperature. Addition of hydrogen
peroxide also significantly increases the corrosion of stainless steel. Exposure
of the reactor to open air between experiments is also found to be a
contributing factor to the corrosion of SCWO experiment.
3)、Pyrethrin
exraction from pyrethrum flowers using carbon dioxide
Extractions of pyrethrins from ground pyrethrum
flowers, using supercritical carbon dioxide as the solvent, were carried out in
a semi-batch pilot plant (extraction volume: 200 ml). Extracts were very similar
to those obtained by hexane Soxhlet extraction, except that the ratio of
pyrethrins I to pyrethrins II was lower (1.58 instead of 1.79), and less
pigments were present. At 40 °C, the amount of crude pyrethrum extract was
found to be independent of pressure above 100 bar. Pyrethrin content in the
crude extract was shown to be higher at 20 °C than at 40 °C and
decreased with decrease in pressure. Effect of particle size was investigated
and the biggest particles yielded a lower quantity of extract and contained less
pyrethrin. Extract obtained from small particles, at 40 °C, contained more
undesired product. We also established that the seed part of the whole flower
contained more crude extract and pyrethrins than the flower part. Pre-treatment,
by preliminary SC-CO2 washing of unground flowers, improved the
quality of SC-CO2 extract, because a part of the undesired waxes was
eliminated by this pre-treatment.
4)、Isolation
of brandy aroma by countercurrent supercritical fluid extraction
Optimization of the countercurrent supercritical
fluid extraction (CC-SFE) conditions to obtain high quality brandy aroma
extracts is presented. The main variables that influence CC extraction
selectivity and efficiency have been studied, such as extraction pressure and
temperature and sample flow rate (related to the solvent-to-feed ratio). A
rotatable central composite experimental design is used to optimize the
combination of these experimental. Experiments have been performed with brandy
using a CC-SFE system at pilot plant scale. The beverage is put directly in
contact with the carbon dioxide in a packed column and the extracts are
recovered in two different fractionation cells, where depressurization occurs.
For each experiment, two extracted fractions and a raffinate are obtained and
its aroma characterized by gas chromatography. A statistical study of the data
obtained is performed including analysis of variance (ANOVA), fitting of a
regression model and response surface study. The obtained results allow to know
the variables that clearly influence the process and also show the interest of
CC-SFE as an useful technique to obtain high-value concentrated brandy aroma
extracts.
5)、Improving
the value of rice by-products by SFE
Rice is an excellent source of complex
carbohydrates, fibre (brown rice), protein and vitamins. The bran layers of
brown rice contain protein rich in eight of the essential amino acids, in
addition to calcium, phosphorus, potassium, niacin, fibre, B vitamins, vitamin E
and a natural oil, which in recent studies appears to have a cholesterol
lowering effect. Rice oil contains three different kinds of natural antioxidants
tocopherols and tocotrienols (tocochromanols) and oryzanols (feruloylsteroltype),
and industry recognizes oxidative stability of rice oil. The aim of this
research was to evaluate the use of SFE technology for recovery of by-products
and developmental research in novel conversion processes to manufacture
value-added food products. Conditions were studied to extract oil from products
and by-products of rice processing chain, and to increase the concentration of
antioxidants (tocochromanols and oryzanols) in oil. High pressure and
temperature, compatible with natural products, enable high yield and efficacious
CO2 usage. The extraction conducted at 10000 psi and 80 °C gave
the highest extraction yield, and the initial analyses indicated that the oil
quality is as suitable for human consumption as the traditionally extracted one.
By-products may be valuable sources of antioxidants, and preliminary results
indicate that it is possible to improve extraction conditions for their
enrichment.
6)、Membrane
separations using reverse micelles in nearcritical and supercritical fluid
solvents
The use of reverse micelles coupled with
ultrafiltration membranes for the separation of macromolecules dissolved in the
cores of the reverse micelles using nearcritical and supercritical fluid
solvents is described. This methodology allows one to address the separation of
a wide range of polar molecules greatly extending the type of molecules that can
be separated using only pure supercritical fluids. The solutes to be separated
are initially dissolved in the reverse micellar solution and introduce into the
pressure vessel containing the membrane. The surfactant and water core are
passed through the membrane while the macromolecule selectivity is based on size
and molecular weight. The ability for continuous recycle in an extraction system
is discussed.
The International
Symposium Goal and History on Supercritical Fluids
The International Symposium on Supercritical Fluids is held every
three years with the goal to provide a forum for top scientists and engineers to
present the latest results in Supercritical Fluid science and technology. The 1st International Symposium was held in 1988 in Nice,France and had 380 participants. The 2nd International
Symposium was held in 1991 in Boston USA.and had 225 participants. The
3rd
International Symposium was held in 1994 in Strabourg,France and
had 480 participants distributed
among 34 countries. The 4th
International Symposium was held in 1997 in Sendai Japan. and had 250
participants distributed
among 25 countries.The 5th International Symposium on Supercritical Fluid will be
held in Aplil 9-11,2000
at Atlanta,USA.Chairman:Pr C.A.Eckert,Georgin Institute of Technology,USA.Language:The offcial language of the Symposium is English.Financial Assistance:Please note
that a very limited amount of financial assistance may be available. Priority for assistance will be for promising young researchers
in
the field.
The 4th International Symposium on Supercritical Fluid Poster
Session
Areas
Adsorption -- Chromatography
-- Critical Region Theory and Behavior -- Crystallization(RESS,GAS,technologies)-- Environmental
Applications -- Extraction -- Fractionation-- Industrial Processing and
Equipment -- Industrial Separations -- Design,Seale-up,and Process Simulation -- Industrial Techniques(Rxn/Sep,Extr./Adsorp)-- Mass
Transfer-- Materials Processing -- Polymeric Material Processing -- Polymer
Fractionayion and Phase Bchavior -- Modeling and Correlation -- Molecular
Simulation -- New Phenomena -- Novel Applications -- Process Design,Scaleup,and Safety -- Reactions in
Supercritical Media-- Reactions with Enzymes -- Reactions and Syntheses in Supercritical CO2--
Reactions inSupercritical water --
Separations -- Slution Structure -- Spectroscopy
and
Interactions --Thermodynamics -- Transport properties.
The 5th International Symposium on
Supercritical Fluid
Keynote Sessions will
include the following
| Catalysis | Novel Applications | ||
| Chromatography | Particle | ||
| Cleaning | Pharmaceutical Applications | ||
| Coatings | Phase Equilibria | ||
| Colloids and Interfaces | Polymer Processing | ||
| Crystallization | Polymer Synthesis | ||
| Design and Scale-up | Process Synthesis | ||
| Environmental Applications | Reactions in Supercritical Fluids | ||
| Extractions and Separations | Spectroscopy and Interactions | ||
| Fractionation | Supercritical Water | ||
| Green Chemistry | Thermophysical Properties | ||
| Materials Processing | Transport Phenomena | |
Poters are invited in any area relevant to supercritical fluids.Capita
Role of Water for Reactions in Supercritical Water
Savage, Phillip E.; Akiya, Naoko
The rates of
organic chemical reactions conducted in supercritical water can be sensitive to
the water density, but the causes of density-dependent rates are not always
clear. In some cases, this influence arises from water molecules serving as
reactants, catalysts, or catalyst precursors. In other cases, water is simply a
solvent but its density-dependent properties influence reaction rates. It is
also possible that physical processes (solvent cage effects, water as a
collision partner) are responsible. In this presentation we will survey this
field and document the role of water in different selected organic chemical
reactions in supercritical water. The systems we consider are the oxidation of
simple fuels, the oxidation of phenol, formic acid decomposition, and hydrogen
peroxide dissociation in supercritical water. Experiments, mechanistic modeling,
quantum chemical calculations, and molecular dynamics simulations have been used
to examine the different possible roles of water and, in some cases, to isolate
the influence of water on chemical reaction rates
Highly Selective Chemical Reactions with Supercritical
Fluids
Sako, Takeshi; Sone, Masato; Sugeta, Tsutomu; Inui, Akifumi; Kamizawa, Chiyoshi
Supercritical fluids are used as advanced reaction
solvents to develop chemical reaction processes with high reaction rate and
selectivity. We show 2 kinds of examples which are promising for both
fundamental research and industrial application. One is the synthesis of
carbonate compounds using supercritical carbon dioxide and the other is the alkylation's,
esterification, etherealization using supercritical alcohol without
any catalyst. The cyclic carbonate compound, 4-methyl-1,3-dioxolan-2-one, was
synthesized from 2-methyloxirane and carbon dioxide in the supercritical
homogeneous region of the mixture, but the reaction did not occur in the
vapor-liquid coexisting region. The high reactivity in the supercritical
condition was originated from the high solubility of the supercritical carbon
dioxide to make uniform reaction field. As compared with the conventional
synthesis of the cyclic carbonates in polar liquid solvents such as diethyl formamide or 1-methyl-2-pyrrolidone, the reaction in the supercritical state had
many advantages of the high reaction rate, high selectivity and simplified
process combined with the purification. We investigated the possibility of
supercritical alcohol as an alternative for the hazardous reagent such as alkyl
halide in substitution reactions of aromatic compounds. The alkylation of
aromatic ring using Lewis acid is known as Friedel-Crafts reaction. Comparing
with the conventional Friedel-Crafts alkylation in a liquid solvent, the present
alkylation using supercritical alcohol did not need any catalyst. It was
remarkable that no polyalkylated compounds were detected using supercritical
alcohol, while the conventional reaction gave the complex product mixture of
mono and polyalkylated compounds. In the case of N-alkylation of aniline, high
selectivity of the monoalkylated compound was realized by the use of
supercritical alcohol without any catalyst. On the other hand, dialkylated
compound was
Development of novel fluorinated surfactants for
extraction of proteins
Goetheer, Earl L.V.; Van den Broeke, Leo J.P.; Vorstman, Marius A.G.; Keurentjes,
Jos T.F.
The isolation
of proteins from complex media, e.g. fermentation broth and dilute solutions,
like whey and blood, is in general very difficult. Multi-step separation
processes are in many cases necessary to isolate the desired products. An
efficient and scaleable bioseparation process is fluid - fluid extraction with
reverse micelles. The ability to extract a wide range of polar solutes,
including proteins, from dilute complex aqueous solutions with reverse micelles
in an organic solvent has been well studied [1]. Besides losses of the organic
solvent, the disadvantage of this method is that high amounts of salts are
produced in the different neutralization steps involved. One way to circumvent
these problems is using supercritical fluids instead of organic solvents. In
this project our aim is to develop a one-step separation process for specific
compounds from complex media, thus accomplishing a large reduction in
separations costs. Prime focus will be on the selective extraction of proteins.
Carbon
dioxide in supercritical fluid state can be a useful alternative for the use of
toxic organic solvents. Over the past decade, there has been intense research
into the development of surfactants capable of forming reverse micelles in CO2
in which the hydrophilic head groups form a core and the “CO2-philic”
tails project into the CO2-continuous phase [2]. Novel fluorinated
surfactants have been developed at our laboratory, which are capable of
solubilizing large amounts of water in carbon dioxide. These surfactants were
used to extract the proteins Bovine Serum Albumine and Lactoferrine from an
aqueous phase with high efficiency.
The way the
selectivity of the extraction is influenced by temperature and pressure is
examined by the use of dendrimers as a model for proteins. The use of dendritic
molecules as model components in an extraction process shows that this type of
molecules can be a versatile tool to gain more insight into the uptake behaviour
of solutes by reverse micelles [3].
A process
will be presented in which reverse micelles based on the novel surfactants in
supercritical CO2 are used to extract proteins from aqueous
solutions. The process basically consists of two stages: one extraction and one
regeneration step in which the protein is recovered. The recovery can simply be
achieved by adjusting pressure and/or temperature. From the results preliminary
conclusions are drawn on the capability of the extraction of polar solutes by
reverse micelles in CO2.[1] M. Dekker, R. Hilhorst and C. Laane (1989) Analyt.
Biochem. 178, 217
[2] E.L.V. Goetheer, M.A.G. Vorstman and J.T.F. Keurentjes (1999) Chem. Eng. Sci.,
54, 1589-1596
[3] E.L.V. Goetheer, M.W.P.L. Baars, M.A.G. Vorstman, E.W. Meijer and J.T.F.
Keurentjes (1999) Proceedings 6th meeting on supercritical Fluids, Nottingham,
UK, 507
Understanding the role of Supercritical CO2
on heterogeneous catalysis: Hydroformylation of propylene
Abraham, Martin A; Snyder, Greg
The last
decade has heralded a paradigm shift in the way engineers view environmental
propriety: waste treatment is no longer an acceptable means of dealing with
process wastes. Our research embodies two precepts of green chemistry, the use
of benign solvents and the development of selective catalysts. Several research
groups have investigated the use of homogeneous catalysts for reaction is
supercritical carbon dioxide, modifying traditional organometallic catalysts for
increased solubility in CO2. We have chosen an alternate route toward selective
chemical synthesis, working towards the development of a selective heterogeneous
catalyst. This paper will report on the evaluation of a selective heterogeneous
catalyst for the hydroformylation of propylene in supercritical CO2. Rhodium
supported on SiO2 is used in a batch reactor for experiments completed at 100°C
and over a range of CO2 pressure. The effect of feed composition (including
reaction pressure) on the reaction rate and the selectivity to the normal
product will be described. Detailed mechanistic studies using diffuse
reflectance infrared spectroscopy in supercritical CO2 reveals the presence of
intermediates on the catalyst surface. Temperature programmed experiments
provide further mechanistic information. A kinetic model derived from a proposed
mechanism that is consistent with the experimental evidence is used to describe
the experimental results.
9
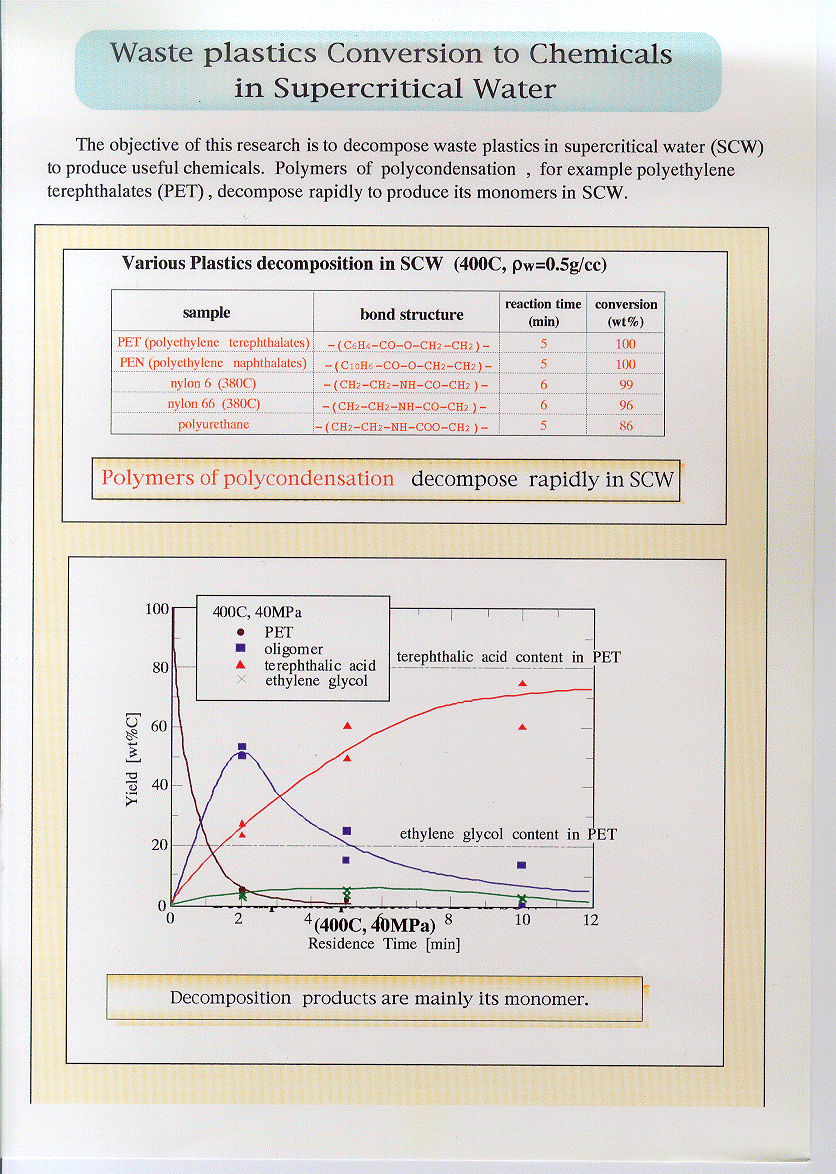
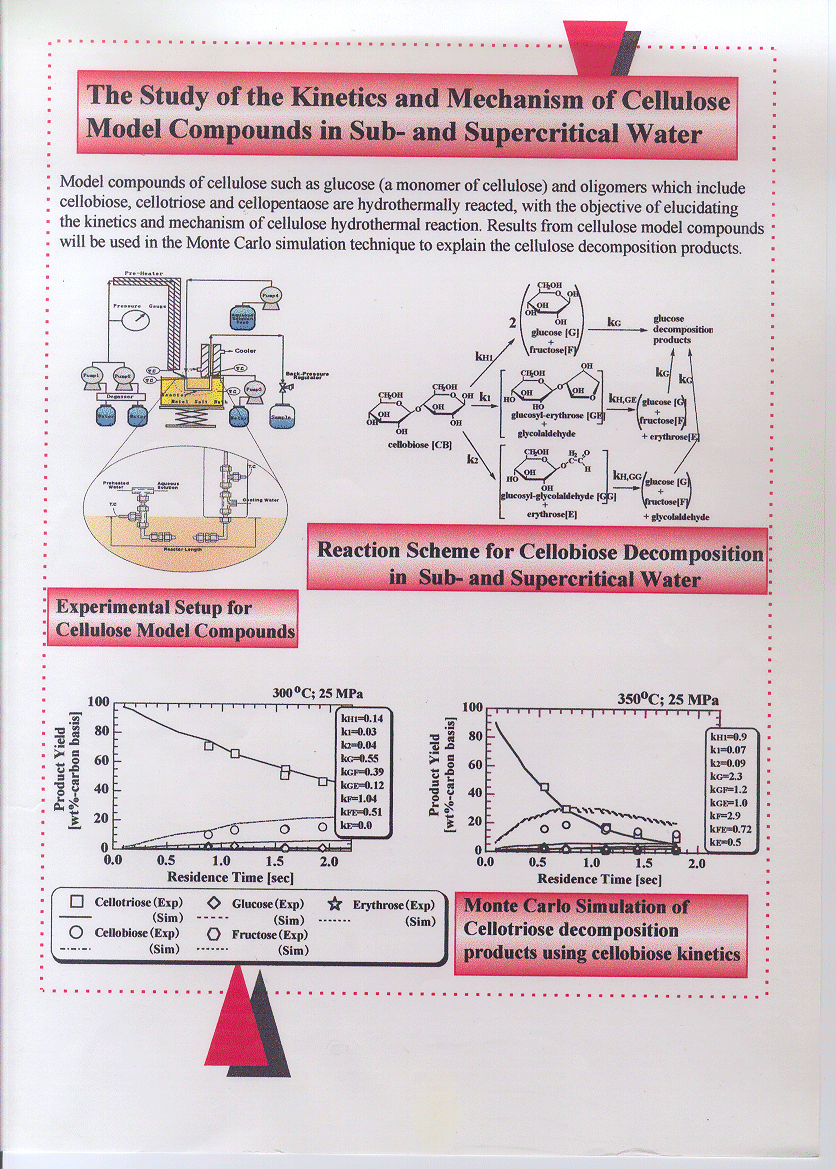
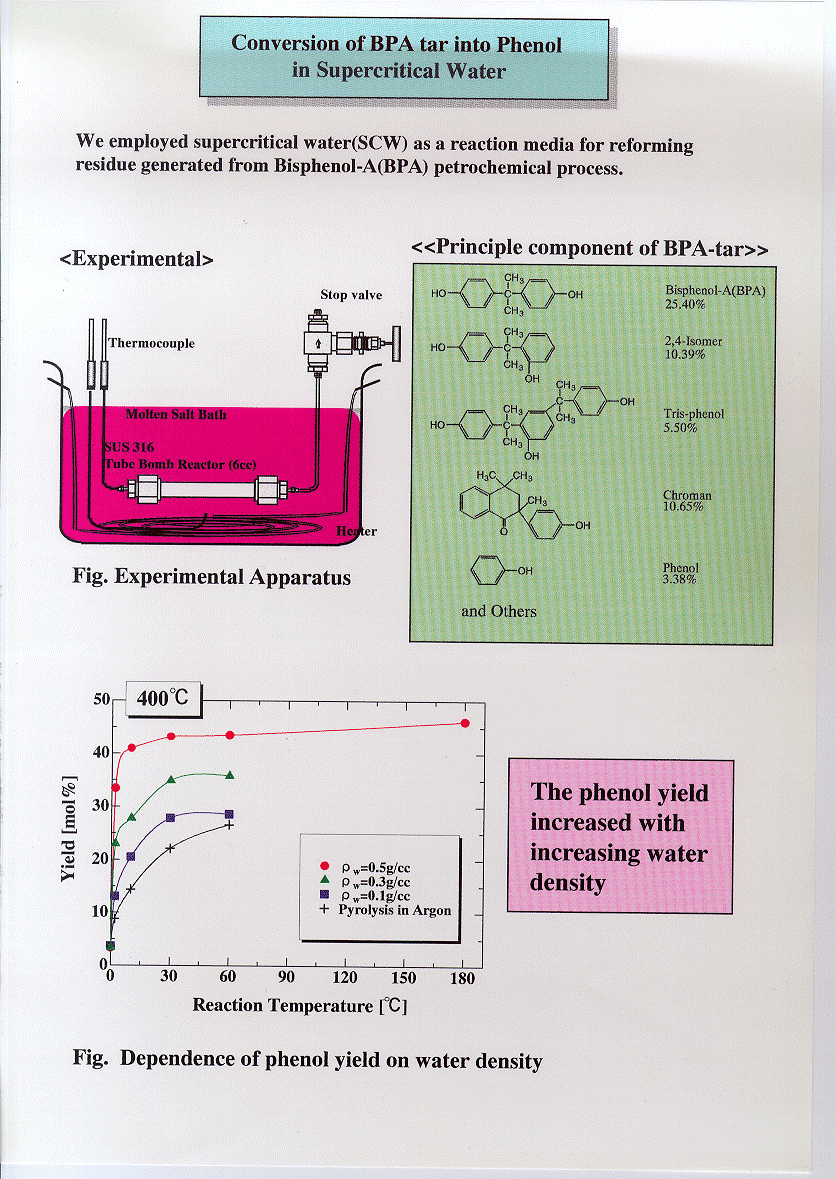
8.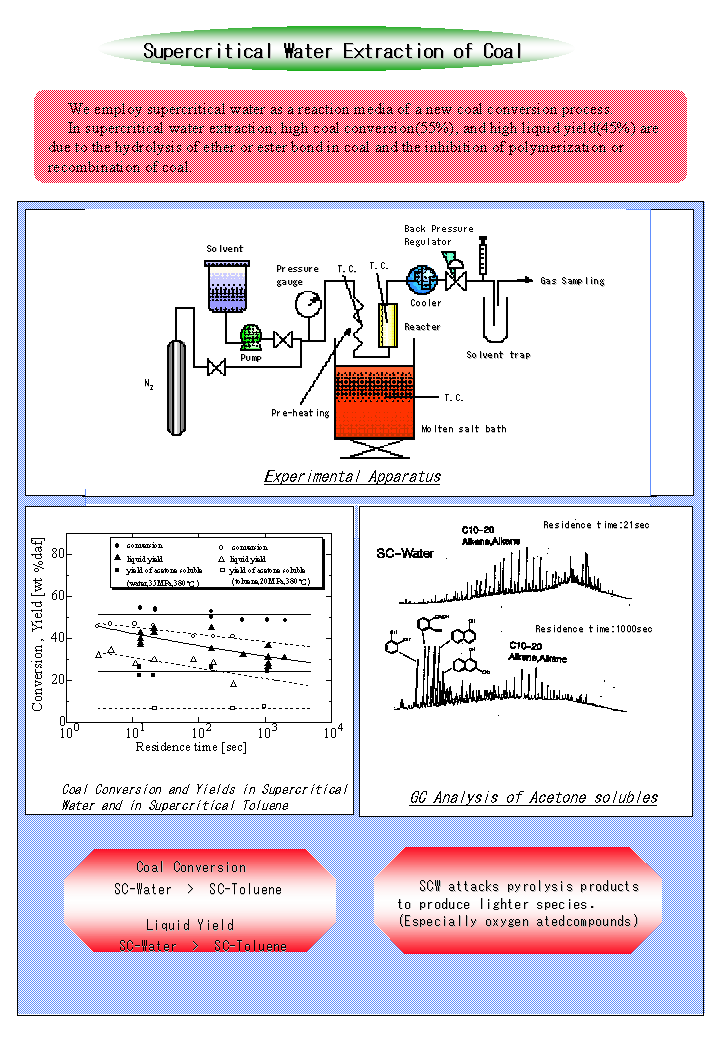
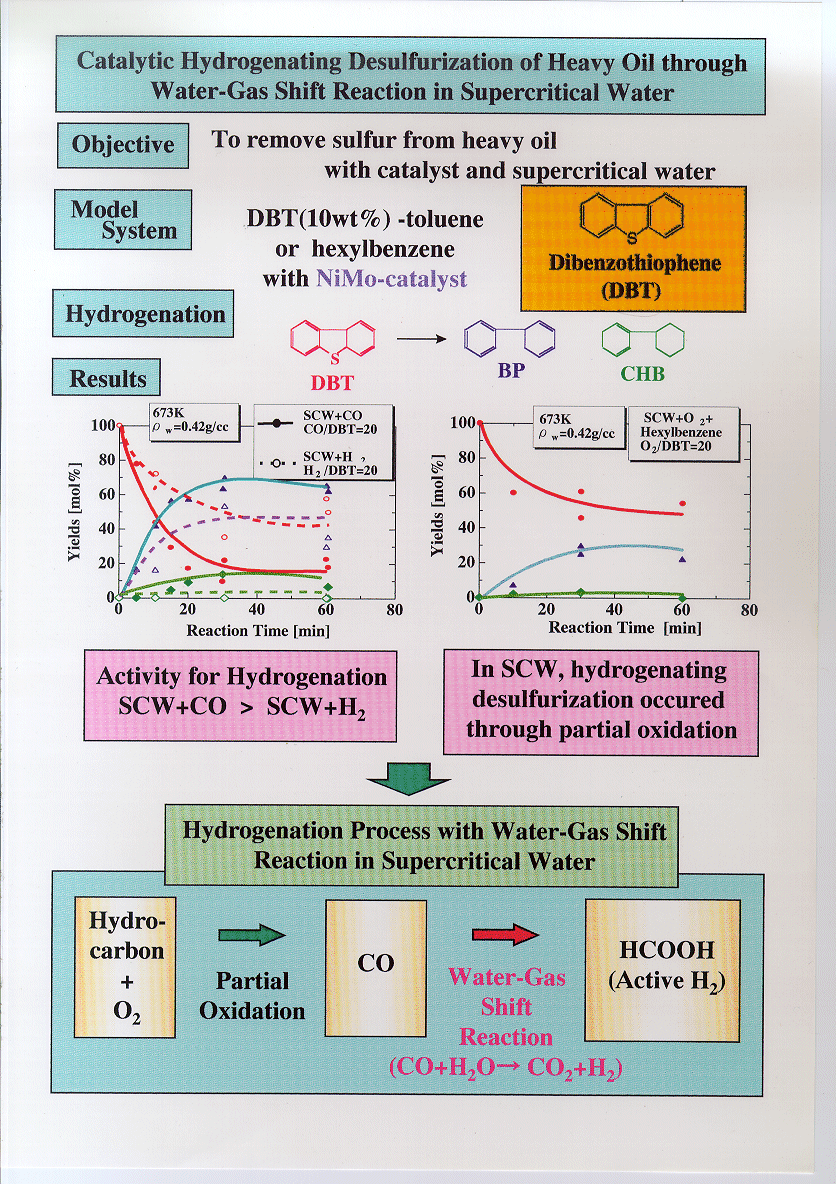
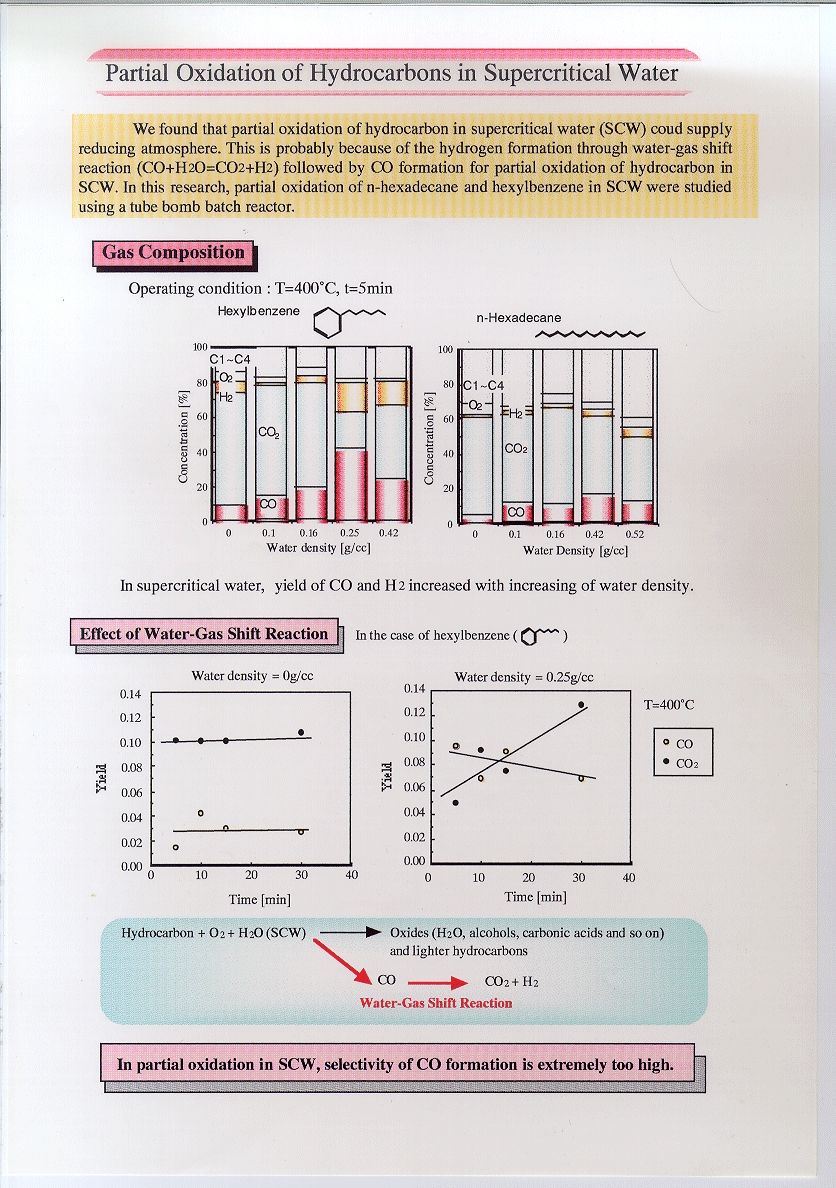
11,