45,815
results found for (Supercritical Fluids 2015-2016)
Dynamical crossover in supercritical
core-softened fluidsOriginal Research Article April 2016Eu. A. Gaiduk, Yu. D.
Fomin, V.N. Ryzhov, E.N. Tsiok, V.V. Brazhkin
Abstract
It is well known
that some liquids can demonstrate an anomalous behavior. Interestingly, this
behavior can be qualitatively reproduced with simple core-softened isotropic
pair-potential systems. Although the anomalous properties of liquids are usually
manifested at low and moderate temperatures, it has recently been recognized
that many important phenomena can appear in supercritical fluids. However,
studies of the supercritical behavior of core-softened fluids have been not yet
reported. In this work, we study dynamical crossover in supercritical
core-softened systems. The crossover line is calculated from three different
criteria, and good agreement between them is observed. It is found that the
behavior of the dynamical crossover line of core-softened systems is quite
complex due to its quasi-binary nature.
Process integration for turmeric
products extraction using supercritical fluids and pressurized liquids: Economic
evaluationOriginal Research Article April 2016,J.
Felipe Osorio-Tobón,
Pedro I.N. Carvalho, Mauricio A. Rostagno, M. Angela A. Meireles
Abstract
An economic
evaluation of an integrated process to produce products derived from turmeric
was performed. Process integration of supercritical fluid extraction (SFE),
pressurized liquid extraction (PLE), and supercritical antisolvent process (SAS)
has been investigated in order to obtain turmeric essential oil (TEO) and
powdered curcuminoid-rich extract (PCE). Scale-up led to a decrease in the cost
of manufacture (COM) for both the products. Volatile oil and powdered
curcuminoid-rich extract COMs decreased from US$ 112.70 kg−1 to US$ 85.58 kg−1
and from US$ 174.80 kg−1 to US$ 141.63 kg−1, respectively, when a raw material
costing US$ 7.27 kg−1 was used, and the capacity of the system increased from 2×
50 L to 2×
500 L. The raw material cost had a significant effect on the process expenses.
When the capacity of the system was 2×
50 L, and the raw material cost decreased from US$ 7.27 kg−1 to US$ 1.59 kg−1,
the COM of volatile oil and powdered curcuminoid-rich extract decreased from US$
112.70 kg−1 to US$ 64.97 kg−1 and from US$ 174.80 kg−1 to US$ 140.96 kg−1,
respectively. The economic evaluation results of this study clearly demonstrate
that the integrated process is a feasible alternative and an attractive option
to produce derivatives from turmeric.
Asymmetric catalytic synthesis of functionalized
tetrahydroquinolines in supercritical fluidsOriginal Research Article March
2016, Evgeniya V. Filatova, Olga V. Turova, Ilya V. Kuchurov, Alexey A.
Kostenko, Albert G. Nigmatov, Sergei G. Zlotin
Abstract
Bifunctional
tertiary amine-catalysed Michael-initiated asymmetric domino-reactions are
accomplished for the first time in supercritical fluids (sc-CO2 and sc-CHF3). In
the proposed conditions, o-N-tosylaminophenyl
α,β-unsaturated
ketones react with α-nitroalkenes
to afford valuable to pharmacology densely functionalized chiral
tetrahydroquinolines in moderate to high yields and with very high diastereo-
(dr > 99:1) and enantioselectivity (up to 98% ee).
Fabrication of high aspect ratio copper nanowires using supercritical CO2 fluids electroplating technique in AAO templateOriginal Research Article, April 2016, Ho-Chiao Chuang, Guan-Yi Hong, Jorge Sanchez
Oxidative dehydrogenation of isobutane
catalyzed by an activated carbon fiber cloth exposed to supercritical
fluidsOriginal Research Article,August
2015, Nicolas Martin-Sanchez, O.S.G.P. Soares, Manuel F.R. Pereira, M.Jesus
Sanchez-Montero, José
L. Figueiredo, Francisco Salvador
Abstract
Modifications
that supercritical fluids promote in the surface chemistry of carbon materials
are scarcely investigated. Due to this lack of knowledge, carbon materials
exposed to supercritical fluids have not been tested in any catalytic
application. Here, we present the oxidative dehydrogenation of isobutane
catalyzed by an activated carbon fiber cloth previously exposed to supercritical
carbon dioxide, supercritical water and nitric acid. The role of carbonyl–quinone
groups as active sites is confirmed by their direct correlation with catalytic
activity. The ability of the active sites to produce isobutene is hindered by
the presence of acidic groups. Supercritical treatments develop microporosity,
while removing acidic oxygen surface groups and incorporating carbonyl–quinone
groups, so they are appropriate methods to develop efficient carbon catalysts
for this reaction. Coke deposits formed during reaction modify the surface
chemistry and porosity of the catalysts. Samples presenting high surface areas
are deactivated by coke deposition more slowly. Thanks to it, fibers exposed to
supercritical water showed the best performance.
Chapter 3 - Biocatalysis in Organic
Solvents, Supercritical Fluids and Ionic Liquids, 2016, Chen Cao, Tomoko Matsuda
Abstract
Biocatalysis in
nonaqueous solvents has promoted green chemistry by controlling reactions as
well as by simplifying the work-up procedure. Biocatalysis in nonaqueous
solvents such as organic solvents, supercritical fluids, and ionic liquids is
described in this chapter to provide theoretical information and practical
examples. Biocatalysis in biphasic solvent systems comprising aqueous
–
organic, aqueous –
supercritical fluids, aqueous
–
ionic liquids and ionic liquids
–
supercritical fluids are also discussed.
Revisiting complete miscibility between
silicate melts and hydrous fluids, and the extreme enrichment of some elements
in the supercritical state —
Consequences for the formation of pegmatites and ore depositsOriginal Research
Article, January 2016, R. Thomas, P. Davidson
Abstract
Using water and
major and trace element data obtained from melt inclusions primarily in
pegmatite quartz we have shown in this, and previous papers, that melt–melt–fluid
immiscibility is widespread and an important process during the generation of
granitic pegmatites. Furthermore we have shown that the formation of pegmatites
actually begins in the supercritical melt/fluid stage. In this study we extend
previous work, and in particular explore the behavior of 10 different elements
(Be, F, P, S, Cl, As, Rb, Sn, Cs, and Ta) in the supercritical range. From
preliminary studies we suggest that other elements such as B, Na, K, Zn, Nb, and
Sb will behave similarly near the solvus crest of a generalized pseudo-binary
melt–water
system. This provides important evidence that pegmatite-forming processes have
already begun at high temperatures, in the range of 850–750
°C,
and that may require a re-think on the partitioning behavior of metals in late
stage residual melts exsolved from granitic magmas. The existence of this
parental supercritical silicate melt/fluid, although derived from granitic
magmas, imposes significant constraints of its own, so drawing conclusions about
pegmatite-forming processes from data gained from slightly modified granite
compositions becomes highly problematic.
We propose that the extremely high concentration of some elements over a small
but well defined water concentration range, symmetrically distributed around the
critical point of the pseudo-binary solvus, must be derived from the unusual
properties of the near-critical or supercritical phases. These unusual
properties include extremely low density, viscosity and surface tension, and
high diffusivity, reactivity and mobility. These are exactly the qualities that
make such supercritical fluids an excellent medium for the formations of
pegmatites by reactions with the existing matrix melt + crystal mush, and for
the extreme enrichment of some ore-forming elements, highly soluble in alkali-
and water-rich supercritical fluids.
A very important point in mineral crystallization is the non-uniform local
enrichment of rare and economically significant elements at specific locations
in pegmatites, granites and other rocks. Given the properties of the
supercritical phase identified from melt inclusions the process of Ostwald
ripening can operate very effectively. The driving force for Ostwald ripening
arises because the concentration of solute in the vicinity of small crystals or
clusters is greater than, and in the vicinity of larger crystals or clusters
less than the average supersaturation. The solute in the supercritical fluid
therefore flows from the small crystals to the larger crystals, and permits the
growth of very large crystals at the expense of smaller ones, this is favored by
the extraordinary properties of supercritical fluids. Given the low surface
tension the supercritical fluid can move also through grain boundaries and small
channels or dislocation pipes, aiding incompatible element transport.
Additionally, their consequences for the formation of some important
granite-related ore deposits are briefly outlined.
Shale gas and non-aqueous fracturing
fluids: Opportunities and challenges for supercritical CO2Original Research
Article,June
2015,Richard S. Middleton, J. William Carey, Robert P. Currier, Jeffrey D.
Hyman, Qinjun Kang, Satish Karra, Joaquín
Jiménez-Martínez,
Mark L. Porter, Hari S. Viswanathan
Abstract
Hydraulic
fracturing of shale formations in the United States has led to a domestic energy
boom. Currently, water is the only fracturing fluid regularly used in commercial
shale oil and gas production. Industry and researchers are interested in
non-aqueous working fluids due to their potential to increase production, reduce
water requirements, and to minimize environmental impacts. Using a combination
of new experimental and modeling data at multiple scales, we analyze the
benefits and drawbacks of using CO2 as a working fluid for shale gas production.
We theorize and outline potential advantages of CO2 including enhanced
fracturing and fracture propagation, reduction of flow-blocking mechanisms,
increased desorption of methane adsorbed in organic-rich parts of the shale, and
a reduction or elimination of the deep re-injection of flow-back water that has
been linked to induced seismicity and other environmental concerns. We also
examine likely disadvantages including costs and safety issues associated with
handling large volumes of supercritical CO2. The advantages could have a
significant impact over time leading to substantially increased gas production.
In addition, if CO2 proves to be an effective fracturing fluid, then shale gas
formations could become a major utilization option for carbon sequestration.
Improved dissolution and bioavailability
of silymarin delivered by a solid dispersion prepared using supercritical
fluidsOriginal Research Article,June
2015, Gang Yang, Yaping Zhao, Nianping Feng, Yongtai Zhang, Ying Liu, Beilei
Dang
Abstract
The objective of
this study was to improve the dissolution and bioavailability of silymarin (SM).
Solid dispersions (SDs) were prepared using solution-enhanced dispersion by
supercritical fluids (SEDS) and evaluated in vitro and in vivo, compared with
pure SM powder. The particle sizes, stability, and contents of residual solvent
of the prepared SM-SDs with SEDS and solvent evaporation (SE) were investigated.
Four polymer matrix materials were evaluated for the preparation of SM-SD-SEDS,
and the hydrophilic polymer, polyvinyl pyrrolidone K17, was selected with a
ratio of 1:5 between SM and the polymer. Physicochemical analyses using X-ray
diffraction and differential scanning calorimetry indicated that SM was
dispersed in SD in an amorphous state. The optimized SM-SD-SEDS showed no loss
of SM after storage for 6 months and negligible residual solvent (ethanol) was
detected using gas chromatography. In vitro drug release was increased from the
SM-SD-SEDS, as compared with pure SM powder or SM-SD-SE. In vivo, the area under
the rat plasma SM concentration-time curve and the maximum plasma SM
concentration were 2.4-fold and 1.9-fold higher, respectively, after oral
administration of SM-SD-SEDS as compared with an aqueous SM suspension. These
results illustrated the potential of using SEDS to prepare SM-SD, further
improving the biopharmaceutical properties of this compound.
A high-pressure high-temperature setup
for in situ Raman spectroscopy of supercritical fluidsOriginal Research Article,
May 2015, Marion Louvel, Amélie
Bordage, Cécile
Da Silva-Cadoux, Denis Testemale, Eric Lahera, William Del Net, Olivier
Geaymond, Jean Dubessy, Roger Argoud, Jean-Louis Hazemann
Abstract
A
high-pressure/high-temperature cell dedicated to Raman spectroscopy is
presented. The P and T ranges are optimized for the study of supercritical
fluids (T ≤
975 K, P ≤
200 MPa); the P and T parameters are controlled independently. The autoclave was
adapted from a previous cell (designed for X-ray absorption and scattering
spectroscopies) to answer the specific requirement of Raman spectroscopy.
Original high pressure windows and sample cell have been developed to ensure
both pressure resistance and transparency for the optical laser and Raman
scattering beams. An optical integration of the autoclave in the Raman
spectrometer setup was designed to perform in situ Raman experiments on fluids.
The spectroscopic measurements can be combined with visual observation, which is
particularly useful for the study of multi-phasic systems. As examples of the
efficiency of the set-up and its scientific opportunities, we present Raman
measurements on water, carbon dioxide and mixed H2O–CO2
system obtained from ambient to supercritical conditions.
Growth factors delivery from hybrid
PCL-starch scaffolds processed using supercritical fluid technologyOriginal
Research Article,May
2016, Luis Diaz-Gomez, Angel Concheiro, Carmen Alvarez-Lorenzo, Carlos A. García-González
Close abstractGraphical abstractResearch highlights Purchase PDF - $35.95
Supplementary content
Abstract
Synthetic
polymeric scaffolds to be used as surrogates of autologous bone grafts should
not only have suitable physicochemical and mechanical properties, but also
contain bioactive agents such as growth factors (GFs) to facilitate the tissue
growth. For this purpose, cost-effective and autologous GFs sources are
preferred to avoid some post-surgery complications after implantation, like
immunogenicity or disease transmission, and the scaffolds should be processed
using methods able to preserve GFs activity. In this work, poly(ɛ-caprolactone)
(PCL) scaffolds incorporating GFs were processed using a green foaming process
based on supercritical fluid technology. Preparation rich in growth factors
(PRGF), a natural and highly available cocktail of GFs obtained from platelet
rich plasma (PRP), was used as GF source. PCL:starch:PRGF (85:10:5 weight ratio)
porous solid scaffolds were obtained by a supercritical CO2-assisted foaming
process at 100 bar and 37
°C
with no need of post-processing steps. Bioactivity of GFs after processing and
scaffold cytocompatibility were confirmed using mesenchymal stem cells. The
performance of starch as GF control release component was shown to be dependent
on starch pre-gelification conditions.
High performance by N-doped graphene via
supercritical fluid processing using an oxime nitrogen sourceOriginal Research
Article,March
2016,S.Suresh
Balaji, A. Elavarasan, M. Sathish
Abstract
Heteroatom doped
graphene has been proved for its promising applications in electrochemical
energy storage systems. Here, nitrogen (N) doped graphene was prepared via two
different techniques namely supercritical fluid assisted processing and
hydrothermal heat treatment using dimethylglyoxime (DMG) as an oxime nitrogen
precursor. The FT-IR and Raman spectra showed the N-containing functional group
in the graphene. The XRD analysis revealed the complete reduction of graphene
oxide during the supercritical fluid processing. The elemental analysis and
X-ray photoelectron spectroscopy revealed the amount and nature of N-doping in
the graphene, respectively. The surface morphology and physical nature of the
samples were analyzed using scanning and transmission electron microscopic
analysis. The electrochemical performance of prepared electrode materials was
evaluated using cyclic voltammetry, galvanostatic charge-discharge analysis and
electrochemical impedance spectroscopy. The N-doped graphene prepared via
supercritical fluid assisted processing exhibit enhanced capacitive behaviour
with a maximum specific capacitance of 286 Fg−1 at a current density of 0.5 A/g.
The cycling studies showed 98% specific capacity retention with 100% coulombic
efficiency over 1000 cycles at 5 A/g. The enhanced specific capacitance of
N-doped graphene prepared via supercritical fluid heat treatment over the
hydrothermal heat treatment is ascribed to the nature of N-doping in the
graphene.
Prediction of heat transfer to
supercritical fluids by the use of Algebraic Heat Flux ModelsOriginal Research
Article, February 2016, Andrea Pucciarelli, Medhat Sharabi, Walter Ambrosini
Abstract
The paper
discusses capabilities and limitations of Algebraic Heat Flux Models in
predicting heat transfer to supercritical fluids. The model was implemented in a
commercial code and used as a basis for obtaining an advanced definition of the
turbulent Prandtl number and an improved estimate of the buoyancy production of
turbulence kinetic energy. A comparison between the obtained results and
experimental data available in literature is performed highlighting promising
features, in particular when dealing with trans-pseudo-critical conditions.
Experimental conditions using different fluids where analysed showing
improvements with respect to two-equation turbulence models; a reference DNS
calculation is considered as well for comparison. Calculated wall temperature
values are in general well reproduced by the methodology and sensitivity
analyses show that improvements may be obtained in future works by selecting
case-specific AHFM parameters in association with different turbulence models.
Determination and correlation of
infinite dilution binary diffusion coefficients for aluminum acetylacetonate in
supercritical and liquid fluidsOriginal Research Article,January
2016,Chang Yi Kong, Kou Watanabe, Toshitaka Funazukuri
Abstract
The infinite
dilution binary diffusion coefficients D12 for aluminum acetylacetonate in
supercritical CO2 were determined at 308.15–333.15
K and at 7.80–40.00
MPa by the chromatographic impulse response (CIR) method. And, the diffusion
measurements in liquid ethanol were carried out at 300.15–333.15
K and at 0.10 and 30.00 MPa by the Taylor dispersion method. It was found that
the D12 values measured in supercritical CO2 show slowing down in the region of
near critical point. The determined activation energies of diffusion were 23.0,
13.7, 12.0, 11.3 kJ/mol at 12.00, 20.00, 25.00, 35.00 MPa in supercritical CO2
and 19.1 kJ/mol at 0.10 MPa in liquid ethanol, respectively. All determined 90
diffusion data in this study can be correlated with the equation of D12 [m2/s] =
1.558 ×
10−14 T [K] η
[Pa s]−0.761 with average absolute relative deviation (AARD) of 5.6% over a wide
fluid viscosity range from 2.462
×
10−5 to 1.258 ×
10−3 Pa s.
Microbial Inactivation by Ultrasound
Assisted Supercritical FluidsOriginal Research Article, 2015, Jose Benedito,
Carmen Ortuño, Rosa Isela Castillo-Zamudio, Antonio Mulet
Abstract
A method
combining supercritical carbon dioxide (SC-CO2) and high power ultrasound (HPU)
has been developed and tested for microbial/enzyme inactivation purposes, at
different process conditions for both liquid and solid matrices. In culture
media, using only SC-CO2, the inactivation rate of E. coli and S. cerevisiae
increased with pressure and temperature; and the total inactivation (7-8
log-cycles) was attained after 25 and 140 min of SC-CO2 (350 bar, 36
°C)
treatment, respectively. Using SC-CO2+HPU, the time for the total inactivation
of both microorganisms was reduced to only 1-2 min, at any condition selected.
The SC-CO2+HPU inactivation of both microorganisms was slower in juices (avg.
4.9 min) than in culture media (avg. 1.5 min). In solid samples (chicken, turkey
ham and dry-cured pork cured ham) treated with SC-CO2 and SC-CO2+HPU, the
inactivation rate of E. coli increased with temperature. The application of HPU
to the SC-CO2 treatments accelerated the inactivation rate of E. coli and that
effect was more pronounced in treatments with isotonic solution surrounding the
solid food samples. The application of HPU enhanced the SC-CO2 inactivation
mechanisms of microorganisms, generating a vigorous agitation that facilitated
the CO2 solubilization and the mass transfer process. The cavitation generated
by HPU could damage the cell walls accelerating the extraction of vital
constituents and the microbial death. Thus, using the combined technique,
reasonable industrial processing times and mild process conditions could be used
which could result into a cost reduction and lead to the minimization in the
food nutritional and organoleptic changes.
A new equation of state for gaseous,
liquid, and supercritical fluidsOriginal Research Article,February
2016, N. Farzi, P. Hosseini
Abstract
In the present
study, a new equation of state (EOS) was derived by using the thermodynamic
equation of state and the intermolecular potential (3, 9, 12). It was shown that
the EOS is applicable in low and high ranges of temperature, pressure and
density for gaseous, liquid and supercritical fluids and even in liquid–gas
phase transition region. The new EOS is applicable for a variety of fluids such
as polar, nonpolar, rare gases, short-chain and long-chain hydrocarbon fluids.
The absolute percent deviation of the calculated density for gaseous, liquid and
supercritical fluids is very low. The common bulk modulus point and the common
compression point regularities were predicted by the new EOS. The new EOS was
compared with some equations of state which had been derived similarly. It is
shown that the repulsive potential used in the EOS derivation is effective in
predicting correct fluid properties.
Supercritical fluids as a green
technology for the pretreatment of lignocellulosic biomassOriginal Research
Articl
, January 2016, L.V. Daza Serna, C.E.
Orrego Alzate, C.A. Cardona Alzate
Abstrac
One of the main drawbacks for using
lignocellulosic biomass is related to its recalcitrance. The pretreatment of
lignocellulosic biomass plays an important role for delignification and
crystallinity reduction purposes. In this work rice husk (RH) was submitted to
supercritical pretreatment at 80
°C
and 270 bar with the aim to determine the effect on lignin content,
crystallinity as well as enzymatic digestibility. The yields obtained were
compared with dilute sulfuric acid pretreatment as base case. Additionally a
techno-economic and environmental comparison of the both pretreatment
technologies was performed. The results show a lignin content reduction up to
90.6% for the sample with 75% moisture content using a water–ethanol
mixture. The results for crystallinity and enzymatic digestibility demonstrated
that no reductions were reached. Supercritical pretreatment presents the best
economical and environmental performance considering the solvents and carbon
dioxide recycling.
Formation of zinc oxide thin film using supercritical fluids and its application
in fabricating a reliable Cu/glass stackOriginal Research Article,June
2015, Mitsuhiro Watanabe, Shigeaki Tamekuni, Eiichi Kondo
Abstra
This paper demonstrates zinc oxide
(ZnO) film formation by using supercritical fluid chemical deposition and the
application of the ZnO films in improving the adhesion of Cu/glass stacks.
Bis(2-methoxy-6-methyl-3,5-heptanedionate)zinc(II) was used as a precursor, and
the deposition was carried out at different oxygen contents and temperatures.
When oxygen content was high and/or deposition temperature was high, white
polycrystalline ZnO films were deposited; otherwise, the films were brown and
had a higher carbon content. XPS analyses revealed that the white ZnO films had
less carbon contamination. A significant improvement in the adhesion of the
Cu/glass stacks was observed when the fabricated ZnO film was inserted between
Cu and glass.
Molecular simulations of supercritical fluid
systemsReview Article,February
2016, John M. Stubbs
Abstract
Molecular simulation has become
increasingly common as a means to study properties of pure supercritical fluids
(SCFs) as well as their solutions. With the large number of studies, and wide
range of properties reported, it is advantageous to summarize the current
approaches. To this end, both Monte Carlo and molecular dynamics molecular
simulation methods are briefly introduced together with important points of
molecular models. Subsequently, water and carbon dioxide SCFs are focused on
both neat and in mixtures, and an overview of reported properties, including
select descriptions of the methods used to determine them, is given. This work
serves as a starting point to understanding and carrying out molecular
simulations on SCFs
Bio-based polymers, supercritical fluids
and tissue engineeringReview Article, May 2015,
Aurelio Salerno, Concepción
Domingo Pascual
Abstract
Tissue engineering and health care involve the diagnosis, treatment, and prevention of diseases with the ultimate goal to improve people's quality of life by developing advanced materials and therapeutic approaches based on scaffolds and drug delivery. Bio-based polymers are excellent candidate materials for these purposes as, for example, they provide biophysical and biochemical properties able to induce the correct biological response for both in vitro and in vivo applications. This review aims to provide the reader with an overview of the use of bio-based polymers, as well as their blends and composites, for tissue engineering and health care purposes. Special emphasis is given to supercritical fluid processing of bio-based polymers for drug delivery and scaffold fabrication, as they enable the preparation of biomedical systems and avoid the use of chemicals potentially harmful to cells and living tissues. The last part of this review highlights some significant in vitro and in vivo applications of bio-based polymeric scaffolds for the regeneration of specific tissues, namely bone, cartilage, blood vessel, nerve, skin and dermis.
Scale-up study of supercritical fluid
extraction process for Baccharis dracunculifoliaOriginal Research Article,
January 2016, Julia T. Paula, Ana C. Aguiar, Ilza M.O. Sousa, Pedro M.
Magalhães, Mary A. Foglio, Fernando A. Cabral
Abstract
To evaluate the effects of the bed
geometry on the scale-up of fixed bed extractors and on the cost of
supercritical extracts from Baccharis dracunculifolia leaves (local name in
Brazil: alecrim-do-campo), supercritical CO2 (scCO2) extraction curves at 50
°C
and 300 bar at different heights (H) and bed diameter (D), at H/D ratio ranging
from 1.86 to 5.2 were obtained. The global yield in all experiments was around
4.6%, and the scale-up criterion adopted was maintain constant the solvent flux.
Five phenolic compounds of interest, caffeic acid, p-coumaric acid,
trans-cinnamic acid, artepillin C, and kaempferide were quantified by
high-performance liquid chromatography. The fractions exhibited high kaempferide
levels, which varied from 29.07 to 46.47 mg kaempferide per gram of extract. The
optimal manufacturing cost has been estimated at US $ 171.12/kg at 80 min, which
shows the possibility of obtaining B. dracunculifolia extracts at an industrial
level.
Molecular composition of oxygenated
compounds in fast pyrolysis bio-oil and its supercritical fluid extractsOriginal
Research Article,May
2016, Tingting Cheng, Yehua Han, Yanfen Zhang, Chunming Xu
Abstract
A three-step supercritical CO2
extraction was used for selective fractionation of fast pyrolysis bio-oil.
Oxygen-containing compounds in bio-oil and its fractions were characterized
using infrared spectroscopy (IR), 1H nuclear magnetic resonance spectroscopy
(NMR), gas chromatography–mass
spectrometry (GC–MS)
and negative-ion electrospray ionization (ESI) Fourier transform ion cyclotron
resonance mass spectrometry (FT-ICR MS). With appropriate optimization of
extraction parameters, lipids, hemicellulose, lignin and condensed aromatics
were found enriched in three different fractions. The molecular composition and
compound classes were identified by combined analytical techniques, and the
selectivity of the fractionation was proved. The compound-type-specific
fractionation also facilitated the following characterization and provided
insights into the chemical composition of bio-oil. Results indicated that CO2
supercritical fluid extraction was potential for bio-oil preprocessing.
Fluid phase equilibria and mass transfer
studies applied to supercritical fluid extraction of Illicium verum volatile
oilOriginal Research Article,June
2016, Pages 203-211
Rodrigo Scopel, Caroline Finkler da
Silva, Aline Machado Lucas, José
Jacques Garcez, Alexandre T. do Espirito Santo, Rafael Nolibos Almeida, Eduardo
Cassel, Rubem M.F. Vargas
Abstract
Supercritical fluid extraction (SFE)
is one of the techniques used to obtain Illicium verum volatile oil, however,
solubility of the volatile oil in CO2, one of the most important process
variables from the SFE, is still unknown. In this work, vapor liquid equilibria
experiments (VLE) were conducted in order to verify solubility of the major
compound in the I. verum volatile oil, E-anethole, in CO2. In addition, SFE was
carried with 750 g/h and 1500 g/h of CO2 at 308.15 K and 8000 kPa, 9000 kPa,
10,000 kPa and the antioxidant activity of the volatile oil accessed by the DPPH
method. The VLE experimental data was modeled using the Peng–Robinson
EOS (PR-EOS) with three mixing rules (vdW1, vdW2 and MKP). Moreover, SFE process
was simulated using the equation proposed by Sovová
and solubility data was compared with the values obtained by the PR-EOS.
Natural convection in shear-thinning
fluids: Experimental investigations by MRIOriginal Research Article
, April 2016, Mohamed Darbouli, Christel
Métivier, Sébastien
Leclerc, Chérif
Nouar, Mondher Bouteera, Didier Stemmelen
Abstract
An experimental investigation of the
Rayleigh–Bénard
convection in shear-thinning fluids using MRI technics is presented. The
experimental setup consists on a cylindrical cavity defined by a finite aspect
ratio A = D/d = 6. Qualitative and quantitative results are provided. Flow
structure is determined from velocity mapping for a Newtonian fluid, Glycerol
and for shear-thinning fluids, Xanthan gum aqueous solutions with weight
concentrations ranging from 0.1% to 0.2%. In the case of the Glycerol and the
Xanthan solution at 0.1%, one recovers similar results in terms of criticality
with Rac ≈
1800 and patterns since the convection is characterized by rolls. When the
Xanthan concentration is increased, the critical Rayleigh number is not
modified, however the onset occurs with hexagonal pattern. Because the critical
temperature differences increase with the concentrations due to an increase in
viscosity, one can think that hexagonal patterns are due to variations of
physical properties with temperature (non Oberbeck–Boussinesq
effects). Similarities with some results obtained in the Newtonian case are
highlighted. We have observed a transition from hexagonal patterns to rolls by
increasing the Rayleigh number. This pattern transition is characterized by a
discrepancy in the maximal velocity values. By using shear-thinning fluids,
results show an increase in the intensity of convection compared with the
Newtonian case.
Effect of solvent type and ratio on
betacyanins and antioxidant activity of extracts from Hylocereus polyrhizus
flesh and peel by supercritical fluid extraction and solvent extractionOriginal
Research Article,July
2016, Farahnaz Fathordoobady, Hamed
Mirhosseini, Jinap Selamat, Mohd Yazid Abd Manap
Abstract
The main objective of the present
study was to investigate the effect of solvent type and ratio as well as the
extraction techniques (i.e. supercritical fluid extraction (SFE) and
conventional solvent extraction) on betacyanins and antioxidant activity of the
peel and fresh extract from the red pitaya (Hylocereus polyrhizus). The peel and
flesh extracts obtained by SFE at 25 MPa pressure and 10% EtOH/water (v/v)
mixture as a co-solvent contained 24.58 and 91.27 mg/100 ml total betacyanin,
respectively; while the most desirable solvent extraction process resulted in a
relatively higher total betacyanin in the peel and flesh extracts (28.44 and
120.28 mg/100 ml, respectively). The major betacyanins identified in the pitaya
peel and flesh extracts were betanin, isobetanin, phyllocactin, butyrylbetanin,
isophyllocactin and iso-butyrylbetanin. The flesh extract had the stronger
antioxidant activity than the peel extract when the higher proportion of ethanol
to water (E/W) was applied for the extraction
Assessment of ultra high performance
supercritical fluid chromatography as a separation technique for the analysis of
seized drugs: Applicability to synthetic cannabinoidsOriginal Research Article,April
2016, Stephanie Breitenbach, Walter F. Rowe, Bruce McCord, Ira S. Lurie
Abstract
The recent development of modern
methods for ultra high performance supercritical fluid chromatography (UHPSFC)
has great potential for impacting the analysis of seized drugs. In the
separation of synthetic cannabinoids the technique has the potential to produce
superior resolution of positional isomers and diastereomers.
To demonstrate
this potential we have examined the capability of UHPSFC for the analysis of two
different groups of synthetic cannabinoids. The first group was a mixture of 22
controlled synthetic cannabinoids, and the second group included JWH018 and nine
of its non-controlled positional isomers The clear superiority of UHPSFC over
other separation techniques was demonstrated, in that it was capable of near
baseline separation of all 10 positional isomers using a chiral column. In total
we examined four achiral columns, including Acquity UPC2 Torus 2-PIC, Acquity
UPC2 Torus Diol, Acquity UPC2 Torus DEA and Acquity UPC2 Torus 1-AA (1.7
μm
3.0 ×
100 mm), and three chiral columns, including Acquity UPC2 Trefoil AMY1, Acquity
UPC2 Trefoil CEL1 and Acquity UPC2 Trefoil CEL2 (2.5
μm
3.0 ×
150 mm), using mobile phase compositions that combined carbon dioxide with
methanol, acetonitrile, ethanol or isopropanol modifier gradients. Detection was
performed using simultaneous PDA UV detection and quadrupole mass spectrometry.
The orthogonality of UHPSFC, GC and UHPLC for the analysis of these compounds
was demonstrated using principal component analysis. Overall we feel that this
new technique should prove useful in the analysis and detection of seized drug
samples, and will be a useful addition to the compendium of methods for drug
analysis.
A closer study of methanol adsorption
and its impact on solute retentions in supercritical fluid
chromatographyOriginal Research Article,April
2016, Emelie Glenne, Kristina Öhlén,
Hanna Leek, Magnus Klarqvist, Jörgen Samuelsson, Torgny Fornstedt
Abstract
Surface excess adsorption isotherms
of methanol on a diol silica adsorbent were measured in supercritical fluid
chromatography (SFC) using a mixture of methanol and carbon dioxide as mobile
phase. The tracer pulse method was used with deuterium labeled methanol as
solute and the tracer peaks were detected using APCI-MS over the whole
composition range from neat carbon dioxide to neat methanol. The results
indicate that a monolayer (4 Å) of methanol is formed on the stationary phase.
Moreover, the importance of using the set or the actual methanol fractions and
volumetric flows in SFC was investigated by measuring the mass flow respective
pressure and by calculations of the actual volume fraction of methanol. The
result revealed a significant difference between the value set and the actually
delivered volumetric methanol flow rate, especially at low modifier fractions.
If relying only on the set methanol fraction in the calculations, the methanol
layer thickness should in this system be highly overestimated.
Finally,
retention times for a set of solutes were measured and related to the findings
summarized above concerning methanol adsorption. A strongly non-linear
relationship between the logarithms of the retention factors and the modifier
fraction in the mobile phase was revealed, prior to the established monolayer.
At modifier fractions above that required for establishment of the methanol
monolayer, this relationship turns linear which explains why the solute
retention factors are less sensitive to changes in modifier content in this
region.
Supercritical fluid extraction vs
conventional extraction of myrtle leaves and berries: Comparison of antioxidant
activity and identification of bioactive compoundsOriginal Research Article, July 2016, Paula Pereira, Maria-João
Cebola, M. Conceição Oliveira, M. Gabriela Bernardo-Gil
Abstract
In this work, the antioxidant
capacity of extracts of Portuguese myrtle (Myrtus communis L.) is being studied
over a period of three years. The samples were leaves of myrtle collected at the
flowering stage and berries sampled at an early ripened stage. Supercritical
fluid extraction (SFE) extracts were obtained at 23 MPa, 45
°C
and a CO2 flow of 0.3 kg h−1 using ethanol as co-solvent with a flow rate of
0.09 kg h−1. Hydrodistillation was carried out in a Clevenger type apparatus and
the aqueous phase was extracted with diisopropylether having obtained what is
hereby designated as liquid phase extract (LPE).
The antioxidant
capacity of all the extracts was determined by using three different methods:
the Folin–Ciocalteu,
the Trolox Equivalent Antioxidant Capacity (TEAC) and the Oxygen Radical
Absorbance Capacity (ORAC). The results show that the SFE extracts present a
significantly higher antioxidant capacity. The extracts were characterized and
quantified by HPLC-DAD-MS/MS methods. The bioactive compounds identified in all
the extracts were phenolic acids (only in the LPE extracts), flavonoids and
anthocyanins (only in the SFE extracts). The results indicate that the higher
antioxidant capacity of the SFE myrtle extracts is mainly correlated with the
concentration of flavonol glycosides, the myricetin-O-glycosides.
Fast and sensitive supercritical fluid
chromatography –
tandem mass spectrometry multi-class screening method for the determination of
doping agents in urineOriginal Research Article,April
2016, Lucie Nováková,
Vincent Desfontaine, Federico Ponzetto, Raul Nicoli, Martial Saugy, Jean-Luc
Veuthey, Davy Guillarme
Abstract
This study shows the possibility
offered by modern ultra-high performance supercritical fluid chromatography
combined with tandem mass spectrometry in doping control analysis. A high
throughput screening method was developed for 100 substances belonging to the
challenging classes of anabolic agents, hormones and metabolic modulators,
synthetic cannabinoids and glucocorticoids, which should be detected at low
concentrations in urine. To selectively extract these doping agents from urine,
a supported liquid extraction procedure was implemented in a 48-well plate
format. At the tested concentration levels ranging from 0.5 to 5 ng/mL, the
recoveries were better than 70% for 48–68%
of the compounds and higher than 50% for 83–87%
of the tested substances. Due to the numerous interferences related to isomers
of steroids and ions produced by the loss of water in the electrospray source,
the choice of SFC separation conditions was very challenging. After careful
optimization, a Diol stationary phase was employed. The total analysis time for
the screening assay was only 8 min, and interferences as well as susceptibility
to matrix effect (ME) were minimized. With the developed method, about 70% of
the compounds had relative ME within the range
±20%,
at a concentration of 1 and 5 ng/mL. Finally, limits of detection achieved with
the above-described strategy including 5-fold preconcentration were below 0.1
ng/mL for the majority of the tested compounds. Therefore, LODs were
systematically better than the minimum required performance levels established
by the World anti-doping agency, except for very few metabolites.
A rapid method for the separation of
vitamin D and its metabolites by ultra-high performance supercritical fluid
chromatography–mass
spectrometryOriginal Research Article,April
2016, Firas Jumaah, Sara Larsson, Sofia Essén,
Larissa P. Cunico, Cecilia Holm, Charlotta Turner, Margareta Sandahl
Abstract
In this study, a new supercritical
fluid chromatography–mass
spectrometry (SFC–MS)
method has been developed for the separation of nine vitamin D metabolites
within less than eight minutes. This is the first study of analysis of vitamin D
and its metabolites carried out by SFC–MS.
Six columns of orthogonal selectivity were examined, and the best separation was
obtained by using a 1-aminoanthracene (1-AA) column. The number and the position
of hydroxyl groups in the structure of the studied compounds as well as the
number of unsaturated bonds determine the physiochemical properties and, thus
the separation of vitamin D metabolites that is achieved on this column. All D2
and the D3 forms were baseline separated with resolution values > 1.5. The
effects of pressure, temperature, flow rate and different gradient modes were
studied. Electrospray ionization (ESI) and atmospheric pressure chemical
ionization (APCI) were compared in positive mode, both by direct infusion and
after SFC separation. The results showed that the sensitivity in APCI+ was
higher than in ESI+ using direct infusion. In contrast, the sensitivity in APCI+
was 6-fold lower than in ESI+ after SFC separation. The SFC–MS
method was validated between 10 and 500 ng/mL for all analytes with coefficient
of determination (R2) ≥
0.999 for all calibration curves. The limits of detection (LOD) were found to
range between 0.39 and 5.98 ng/mL for 24,25-dihydroxyvitamin D3 (24,25(OH)2D3)
and 1-hydroxyvitamin D2 (1OHD2), respectively. To show its potential, the method
was applied to human plasma samples from healthy individuals. Vitamin D3 (D3),
25-hydroxyvitamin D3 (25OHD3) and 24,25(OH)2D3 were determined in plasma samples
and the concentrations were 6.6
±
3.0 ng/mL, 23.8 ±
9.2 ng/mL and 5.4 ±
2.7 ng/mL, respectively.
Fast separation of triterpenoid saponins
using supercritical fluid chromatography coupled with single quadrupole mass
spectrometryOriginal Research Article,March
2016, Yang Huang, Tingting Zhang, Haibo Zhou, Ying Feng, Chunlin Fan, Weijia
Chen, Jacques Crommen, Zhengjin Jiang
Abstract
Triterpenoid saponins (TSs) are the
most important components of some traditional Chinese medicines (TCMs) and have
exhibited valuable pharmacological properties. In this study, a rapid and
efficient method was developed for the separation of kudinosides, stauntosides
and ginsenosides using supercritical fluid chromatography coupled with single
quadrupole mass spectrometry (SFC-MS). The separation conditions for the
selected TSs were carefully optimized after the initial screening of eight
stationary phases. The best compromise for all compounds in terms of
chromatographic performance and MS sensitivity was obtained when water (5–10%)
and formic acid (0.05%) were added to the supercritical carbon dioxide/MeOH
mobile phase. Beside the composition of the mobile phase, the nature of the
make-up solvent for interfacing SFC with MS was also evaluated. Compared to
reversed phase liquid chromatography, the SFC approach showed higher resolution
and shorter running time. The developed SFC-MS methods were successfully applied
to the separation and identification of TSs present in Ilex latifolia Thunb.,
Panax quinquefolius L. and Panax ginseng C.A. Meyer. These results suggest that
this SFC-MS approach could be employed as a useful tool for the quality
assessment of natural products containing TSs as active components.
Supercritical fluid chromatography
coupled with in-source atmospheric pressure ionization hydrogen/deuterium
exchange mass spectrometry for compound speciationOriginal Research Article,March
2016,Yunju Cho, Man-Ho Choi, Byungjoo Kim,
Sunghwan Kim
Abstract
An experimental setup for the
speciation of compounds by hydrogen/deuterium exchange (HDX) with atmospheric
pressure ionization while performing chromatographic separation is presented.
The proposed experimental setup combines the high performance supercritical
fluid chromatography (SFC) system that can be readily used as an inlet for mass
spectrometry (MS) and atmospheric pressure photo ionization (APPI) or
atmospheric pressure chemical ionization (APCI) HDX. This combination overcomes
the limitation of an approach using conventional liquid chromatography (LC) by
minimizing the amount of deuterium solvents used for separation. In the SFC
separation, supercritical CO2 was used as a major component of the mobile phase,
and methanol was used as a minor co-solvent. By using deuterated methanol
(CH3OD), AP HDX was achieved during SFC separation. To prove the concept, thirty
one nitrogen- and/or oxygen-containing standard compounds were analyzed by
SFC-AP HDX MS. The compounds were successfully speciated from the obtained
SFC-MS spectra. The exchange ions were observed with as low as 1% of CH3OD in
the mobile phase, and separation could be performed within approximately 20 min
using approximately 0.24 mL of CH3OD. The results showed that SFC separation and
APPI/APCI HDX could be successfully performed using the suggested method.
Chiral separations of cathinone and
amphetamine-derivatives: Comparative study between capillary
electrochromatography, supercritical fluid chromatography and three liquid
chromatographic modesOriginal Research Article,March
2016, Dima Albals, Yvan Vander Heyden, Martin G. Schmid, Bezhan Chankvetadze,
Debby Mangelings
Abstract
The screening part of an earlier
defined chiral separation strategy in capillary electrochromatography (CEC) was
used for the separation of ten cathinone- and amphetamine derivatives. They were
analyzed using 4 polysaccharide-based chiral stationary phases (CSPs),
containing cellulose tris(3,5-dimethylphenylcarbamate) (ODRH), amylose
tris(3,5-dimethylphenylcarbamate) (ADH), amylose
tris(5-chloro-2-methylphenylcarbamate) (LA2), and cellulose
tris(4-chloro-3-methylphenylcarbamate) (LC4) as chiral selectors. After applying
the screening to each compound, ADH and LC4 showed the highest success rate. In
a second part of the study, a comparison between CEC and other analytical
techniques used for chiral separations i.e., supercritical fluid chromatography
(SFC), polar organic solvent chromatography (POSC), reversed-phase (RPLC) and
normal-phase liquid chromatography (NPLC), was made. For this purpose, earlier
defined screening approaches for each technique were applied to separate the 10
test substances. This allowed an overall comparison of the success rates of the
screening steps of the 5 techniques for these compounds. The results showed that
CEC had a similar enantioselectivity rate as NPLC and RPLC, producing the
highest number of separations (9 out of 10 racemates). SFC resolved 7 compounds,
while POSC gave only 2 separations. On the other hand, the baseline separation
success rates for NPLC and RPLC was better than for CEC. For a second
comparison, the same chiral stationary phases as in the CEC screening were also
tested with all techniques at their specific screening conditions, which allowed
a direct comparison of the performance of CEC versus the same CSPs in the other
techniques. This comparison revealed that RPLC was able to separate all tested
compounds, and also produced the highest number of baseline separations on the
CSP that were used in the CEC screening step. CEC and NPLC showed the same
success rate: nine out of ten substances were separated. When CEC and NPLC are
combined, separation of the ten compounds can be achieved. SFC and POSC resolved
eight and three compounds, respectively. POSC was the least attractive option as
it expressed only limited enantioselectivity toward these compounds.
Chapter Thirteen - Electronic Properties
in Supercritical Fluids: The Absorption Spectrum of p-Nitroaniline in
Supercritical Water, 2015, Marcelo Hidalgo Cardenuto, Kaline Coutinho, Benedito
J.C. Cabral, Sylvio Canuto
Abstract
Supercritical fluids (SCFs) are of
great interest for their remarkable physical–chemical
properties. Understanding the properties of SCFs is of industrial and academic
interests. Foreign molecules can act as interesting probes giving information
from the changes induced by the supercritical environment. The electronic
absorption spectrum of p-nitroaniline (pNA) in the environment of supercritical
water has been addressed. The thermodynamic condition is assured by Monte Carlo
simulation, and quantum mechanical calculations are performed on sampled
configurations to obtain the spectrum of pNA in supercritical water. Using the
same thermodynamic condition of the experiment (T = 655 K and
ρ
= 0.12 g/cm3), we analyze the electronic polarization of the p-aniline, the
solute–solvent
hydrogen bonds, and the red shift of the intense charge transfer
π–π*
transition. Compared to normal water, the number of solute–solvent
hydrogen bonds in supercritical water decreases to essentially one-fifth, from
2.9 to 0.6. The calculated spectrum in supercritical water is obtained using
time-dependent CAM-B3LYP/aug-cc-pVDZ and shows a maximum located at 305 nm in
good comparison with the experimental maximum observed at 309 nm. In comparison
to the results in the vapor phase, the experimental red shift of 17 nm is very
well described theoretically with the result of 17
±
3 nm. A discussion is also presented of the inhomogeneous broadening
contribution to the half-width at the absorption maximum.
CFD aided approach to design printed
circuit heat exchangers for supercritical CO2 Brayton cycle applicationOriginal
Research Article, June 2016, Seong Gu Kim, Youho Lee, Yoonhan Ahn, Jeong Ik Lee
Abstract
While most conventional PCHE designs
for working fluid of supercritical CO2 require an extension of valid Reynolds
number limits of experimentally obtained correlations, Computational Fluid
Dynamics (CFD) code ANSYS CFX was used to explore validity of existing
correlations beyond their tested Reynolds number ranges. For heat transfer
coefficient correlations, an appropriate piece-wising with Ishizuka’s
and Hesselgreaves’s
correlation is found to enable an extension of Reynolds numbers. For friction
factors, no single existing correlation is found to capture different
temperature and angular dependencies for a wide Reynolds number range. Based on
the comparison of CFD results with the experimentally obtained correlations, a
new CFD-aided correlation covering an extended range of Reynolds number 2000–58,000
for Nusselt number and friction factor is proposed to facilitate PCHE designs
for the supercritical CO2 Brayton cycle application.
Instrument Modifications that Produced
Reduced Plate Heights <2 with Sub-2 μm
Particles and 95% of Theoretical Efficiency at k = 2 in Supercritical Fluid
ChromatographyOriginal Research Article,March
2016,Terry A. Berger
Abstract
The concept of peak fidelity was
shown to be helpful in modeling tubing and detector cell dimensions. Connection
tubing and flow cell variances were modeled to determine appropriate internal ID’s,
lengths, and volumes. A low dispersion plumbing configuration, based on these
calculations, was assembled to replace the standard plumbing and produced the
reported results. The modifications made were straightforward using commercially
available parts. The full theoretical efficiency of a 3
×
100 mm column packed with 1.8
μm
totally porous particles was achieved for the first time in supercritical fluid
chromatography (SFC). Peak fidelity of > 0.95 was maintained to below k = 2. A
reduced plate height as low as 1.87 was measured. Thus, true
“ultra
high performance”
SFC was achieved, with the results a major improvement from all previous SFC
reports.
Since there
were no efficiency losses, none could be attributed to thermal gradients caused
by the expansion of the fluid over large pressure drops, under the conditions
used. Similarly, changes in diffusion coefficients caused by significant
decreases in density during expansion are apparently balanced by the increase in
linear velocity, keeping the ratio between the diffusion coefficient and the
linear velocity a constant. Changing modifier concentration to change retention
was shown to not be a significant problem. All these issues have been a concern
in the past.
Diffusion
coefficients, and viscosity data needs to be collected at high pressures before
the actual limits of SFC can be discovered
Polymers' ultrafine particles for drug
delivery systems precipitated by supercritical carbon dioxide + organic solvent
mixturesOriginal Research Article, May 2016, Valentina Prosapio, Ernesto
Reverchon, Iolanda De Marco
Abstract
The applicability of supercritical
antisolvent precipitation (SAS) is restricted to hydrophobic substances because
of the very limited solubility of water in CO2 at ordinary SAS operating
conditions (40–60
°C,
10–25
MPa). To overcome this limitation, a technique has been developed, named
expanded liquid antisolvent (ELAS), in which mixtures of supercritical carbon
dioxide (scCO2) and organic solvents, at expanded liquid conditions, are used as
the antisolvent: water solubility is widely enhanced.
In this work,
sodium alginate and polyvinyl alcohol (PVA), two water-soluble polymers, used as
carrier for drug delivery systems, were successfully micronized by ELAS.
Different antisolvent mixtures were used: scCO2 + ethanol, scCO2 + acetone and
scCO2 + isopropyl alcohol. Operating at 15 MPa and 40
°C,
varying the organic co-antisolvent, the co-antisolvent mole fraction and the
concentration of the polymer in the aqueous solution, nanoparticles (with a mean
diameter of about 200 nm), microparticles with smooth surface (with a mean
diameter in the range of 0.9–12.5
μm
for sodium alginate and 2–9
μm
for PVA) and nanostructured microparticles (with a mean diameter of about 11
μm)
were produced. XRD analyses on the processed powders revealed that no
modifications in the polymer structure were induced by ELAS processing. Solvent
residue analyses revealed that the co-antisolvent residue ranged between 50 and
300 ppm depending on the organic solvent used.
Investigation on the fluid selection and
evaporation parametric optimization for sub- and supercritical organic Rankine
cycleOriginal Research Article,February
2016, Heng Xu, Naiping Gao, Tong Zhu
Abstract
ORC (Organic Rankine cycle) is a
promising technology for recovery of low-grade heat. In the previous studies,
different conclusions for working fluid selection criterion can be found and
relatively few work for supercritical ORC has been made. Therefore, this paper
investigates the net power output of ORC utilizing waste flue gas with various
evaporation parameters and 12 working fluids in both subcritical and
supercritical condition. The results indicate that the variation of the net
power output with evaporation pressure is related to the heat source
temperature, and the maximum net power output appears at supercritical condition
rather than subcritical condition if the heat source temperature is about 25–40
°C
higher than the working fluid's critical temperature. Besides, the parametric
optimization is performed, and the most suitable working fluids for various flue
gas inlet temperature of 150–250
°C
have been found. It can be found that the most suitable working fluids have a
critical temperature about 40–65
°C
lower than flue gas inlet temperature, and the optimum condition is always
supercritical. For subcritical ORC, it is better to adopt the working fluids
with low evaporation latent heat and high liquid specific heat to pursue a high
net power output.
Effect of fluid temperature on the
frictional coefficient of supercritical pressure water flowing in adiabatic
horizontal tubesOriginal Research Article, July 2016,Qing Zhang, Huixiong Li,
Weiqiang Zhang, Liangxing Li, Xianliang Lei
Abstract
Based on the analysis on sensitivity
of the frictional coefficient of supercritical pressure water to the fluid
temperature, the conflict of the variation trends of the frictional coefficient
with the fluid enthalpy obtained by different researchers was explained. It is
shown that both concave curves and convex curves are reasonable for the
variation trend of the frictional coefficient with the fluid enthalpy. For the
same case, the variation trend of the frictional coefficient with the fluid
enthalpy might change from concave curves to convex curves, or, from convex
curves to concave curves, when the fluid temperature in the calculation of
frictional coefficient is changed by a value as little as only 0.5–1.0
°C.
The maximum error in the measured fluid temperature is estimated to be large
enough (larger than ±1.0
°C),
and might be the main reason for completely different tendencies of the
frictional coefficient obtained in different studies.
Evaluation of the response surface and
hybrid artificial neural network-genetic algorithm methodologies to determine
extraction yield of Ferulago angulata through supercritical fluidOriginal
Research Article, March 2016, Gholamhossein Sodeifian, Seyed Ali
Sajadian, Nedasadat Saadati Ardestani
Abstract
In this research, the influence of
operational parameters such as pressure, temperature, particle size and dynamic
time on the extraction of Ferulago angulata via supercritical carbon dioxide was
investigated. Two models, response surface method (RSM) and artificial neural
network (ANN), were applied for modeling and predicting of the extraction yield.
The capability and sensitivity analysis of two methodologies was evaluated by
some statistical parameters including, correlation coefficients (R2), mean
square error (MSE), and absolute average relative deviation (AARD). The error
values of RSM model (R2 = 0.9645, MSE = 0.0032 and AARD = 3.39) and ANN model
(R2 = 0.9971, MSE = 0.0014 and AARD = 1.32) were calculated. Both models showed
good agreement with the experimental data, but ANN model was more accurate than
the RSM model. RSM and ANN-GA models were subsequently utilized to determine
optimal operating conditions in order to gain the maximum yield of Ferulago
angulate extraction. The essential oil extraction yield in the optimal
conditions was estimated to be 0.851 w/w% and 0.863 w/w% by the RSM and ANN-GA
models, respectively. Assessment and evaluation of the above mentioned models
were carried out through performing triplicate experiments and finally the
average experimental extraction yield was achieved to be 0.867
±
0.004 w/w% at the best operating conditions 20 MPa, 35
°C,
0.742 mm and 101.3 min.
Ecofriendly pretreatment of grey cotton
fabric with enzymes in supercritical carbon dioxide fluidOriginal Research
Article, May
2016, Shi-Qi Liu, Zong-Yao Chen, Jian-Ping Sun, Jia-Jie Long
Abstract
A novel and Ecofriendly process was
developed at the first time for the pretreatment of grey cotton fabric by
employing enzymes with microemulsion in supercritical carbon dioxide fluid
(SCF-CO2). The results show that high efficiency of impurity removal and
significant improvement in wettability of treated substrate were achieved with
the supposed bioprocess. The fabric weight loss and capillary rise height of
substrate were notably influenced by system temperature and pressure, the
dosages of enzymes and sodium bis (2-ethylhexyl) sulfosuccinate (AOT), the
presence of water and initial pH value, as well as treatment time. An optimized
enzymatic and Ecofriendly pretreatment process for desizing and scouring of grey
cotton was recommended in supercritical carbon dioxide media with a system
temperature at 50.0 °C
and a pressure at 13.0 MPa for 60.0 min in the presence of small amounts of
ethanol, water, several enzymes and AOT in the reactor at an initial pH value of
7.0–8.0.
Moreover, the feasibility and the efficiency of the supposed supercritical
bio-pretreatment process were further validated by scanning electronic
microscopy (SEM) analysis.
Patent of cn
Technique and industrial device for refining rice by supercritical polybasic fluid
|
Inventor(s): [CN]; |
Abstract of CN101554211 (A)
The invention belonges to the field of refining rice by supercritical polybasic
fluid. The invention is characterized in that the supercritical polybasic fluid
industrial device with carbon-dioxide zero emission, with pressure less than 22
MPa and temperature less than 40 DEG C, pre-breaks wall of brown rice layer to
be directly prepared as high-nutrient rice; meanwhile, harmful bacteria, mould
and ova are killed, so that freshness date is prolonged and storage loss is
reduced; impurities in the brown rice such as pesticide residue, heavy metal and
dust are removed via ultrasonic and microwave extraction; the brown rice becomes
good-looking and delicious by colouring and aromatizing; besides,
anti-free-radical, anti-aging, anti-radiation and immunity-improving natural
bioactive substances are uniformly added so as to improve health-care function
and added value of the rice, further; thus, fresh and delicious rice with
advantages of high nutrient, water saving, washing prevention and rapid cooking
is produced, with yield being increased by more than 25%; the invention is
executed and popularized widely in rice planting regions, thereby ensuring
national food safety and international food safety.
Process for extracting active ingredients of traditional Chinese medicine in
unit and combined type and device thereof
|
Inventor(s): [CN]; |
Abstract of CN101530674 (A)
The invention belongs to highly effective extraction and separation of the
active ingredients in field of modernization of Chinese medicine and is
characterized in that on the basis of an extraction process and a device
consisting of the combination of 3 novel chemical engineering unit operations of
water percolation distillation, extractive distillation, and highly efficient az
dist azeotropic distilation, the traditional and advanced techniques and devices
for water distillation extraction, extraction by semi-bionic method, extraction
by ultrasonic waves, extraction by enzyme process, extraction by microbial
fermentation and extraction by microwave are preferably selected to constitute
the unit and combined type extraction process and the device thereof; the
process and the device of the invention is especially suitable for extracting
the majority of single Chinese medicine or compound Chinese medicine with both
volatile and non-volatile components; in addition the device operates stably
with stable parameters; compared with the traditional multifunctional
traditional Chinese medicine extracting tank, the tank of the invention features
an extraction rate above 95% and energy conservation by over 50%, contraction of
the production period by over 50%, reduction of production cost per unit product
by 50% and stable content of active ingredients and product quality.
Supercritical fluid drying equipment
Abstract of CN105202888 (A)
The invention relates to supercritical fluid drying equipment which comprises a
drying body, a filter cavity, a first pipeline and a second pipeline. The drying
body comprises a gas drying cavity formed in the upper layer and a heating
cavity formed in the lower layer. The bottom layer of the gas drying cavity is
obliquely arranged, and a flow channel communicated with the heating cavity is
arranged at the bottom of the inclined end. A flow speed induction point, a flow
speed control valve and a control valve handle connected with the flow speed
control valve are arranged in the flow channel. The control valve handle is
arranged on the outer side wall of the drying body.
The supercritical fluiddrying equipment comprises the drying body, and is
reasonable in structure, good in drying and purifying effect and convenient to
maintain.
Supercritical fluid opposite-spraying dyeing device
Abstract of CN105200686 (A)
The invention relates to the field of textile dyeing devices, in particular to a supercritical fluid opposite-spraying
dyeing device, consisting of a cleaning unit, an opposite-spraying dyeing unit,
a rinsing unit, reflux devices, working fluid air
seal units, air seal units, a connection plate, a sealing box, a sealing element
and a pressure regulator valve; according to a supercritical fluid opposite
spraying dyeing process flow, the air seal unit, the working fluid air
seal unit, the reflux device, the cleaning unit, the reflux device, the working fluid air
seal unit, the reflux device, the opposite-spraying dyeing unit, the reflux
device, the working fluid air
seal unit, the reflux device, the rinsing unit, the reflux device, the working fluid air
seal unit and the air seal unit are arranged in sequence. Supercritical fluid is
sprayed to a dyed object from two sides at the same time, the jetting forces of
the fluid are
offset as 0, the dyeing is uniform, and deformation and tension damage of the
dyed object caused by high pressure jetting are eliminated; a level-by-level
pressure reducing air seal structure is adopted, so that continuous dyeing is
realized, and the device is simple and compact in structure and excellent in
manufacturability.
Loquat enzyme nutrient solution and preparation method thereof
Abstract of CN105193989 (A)
The invention discloses a loquat enzyme nutrient solution and a preparation
method thereof and relates to the technical field of biological fermentation
solutions. The loquat enzyme nutrient solution comprises raw materials including
loquats, dried loquat leaves, honey, oligosaccharide, dried yeast and water. The
preparation method comprises steps as follows: the loquats are subjected to pulp
making firstly, the dried loquat leaves are boiled with water, are cooled and
then are uniformly mixed with the honey, the oligosaccharide and the dried
yeast, water is added, and the mixture is uniformly mixed and placed in a
container for sealing fermentation; finally, acquired fermentation broth is
processed and extracted with an supercritical fluid extraction
and separation technology, and the loquat enzyme nutrient solution is obtained.
According to the loquat enzyme nutrient solution and the preparation method
thereof, the safe loquat enzyme nutrient solution which is free of side effects
and can be taken as a beverage for drinking is prepared with lower cost and the
simple method and has remarkable cough relieving and sputum reducing effects.
Purslane extract and preparation method thereof
Abstract of CN105193876 (A)
The invention belongs to the technical field of traditional Chinese medicines or
cosmetics, and particularly relates to a purslane extract and a preparation
method thereof. The extract is prepared by the following steps: drying taken
purslane; with 95% ethanol as an entrainer, carrying out CO2 supercritical fluid extraction,
adding 1,3-butanediol to an extract, completely recovering a reagent from an
organic layer, and drying the reagent to obtain an extract A; carrying out
enzyme hydrolysis on residues after supercritical extraction,
adding water to extract, filtering and adding activated carbon and clay to the
extract liquid, centrifuging and drying the extract liquid to obtain an extract
B; and mixing the extract A with the extract B evenly. According to the
preparation method provided by the invention, the extract A is rich in
alpha-linolenic acid. The polysaccharide content of the extract B is high.
Compared with the purslane extract in the prior art, the purslane extract
obtained by the extraction method has better antibacterial, anti-inflammatory
and anti-irritation effects.
Navel orange enzyme nutrient solution and preparation method thereof
Abstract of CN105192830 (A)
The invention discloses a navel orange enzyme nutrient solution and a
preparation method thereof and relates to the technical field of biological
fermentation solutions. The navel orange enzyme nutrient solution comprises raw
materials including navel oranges, dried liquorice, honey, oligosaccharide,
dried yeast and water. The preparation method comprises steps as follows: the
navel oranges are subjected to pulp making firstly, the dried liquorice is
boiled with water, is cooled and then is uniformly mixed with the honey, the
oligosaccharide and the dried yeast, water is added, and the mixture is
uniformly mixed and placed in a container for sealing fermentation; finally,
acquired fermentation broth is processed and extracted with an supercritical fluid extraction
and separation technology, and the navel orange enzyme nutrient solution is
obtained. According to the navel orange enzyme nutrient solution and the
preparation method thereof, the safe navel orange enzyme nutrient solution which
is free of side effects and can be taken as a beverage for drinking is prepared
with lower cost and the simple method, can replace western medicine, effectively
assists a body in automatic regulation of blood pressure and can be used for
reducing cholesterol, and hypertension, hyperglycemia and hyperlipemia can be
relieved and repaired when the human body is properly supplemented with the
orange enzyme.
High-power drafting flame-retardant post-processing method for high-tenacity
polyester in supercriticalCO2
Abstract of CN105177769 (A)
The invention relates to a high-power drafting flame-retardant post-processing
method for high-tenacity polyester in supercritical CO2,
which comprises the following steps: adding a flame retardant into a closed
container, exhausting air at 80-120 DEG C, feeding CO2, increasing the pressure
in the container to 8-13MPa to obtain CO2 supercritical fluid,
and soaking the primary filament of high-tenacity polyester in the supercritical fluid for
swelling; and after swelling, carrying out high-power drafting on the primary
filament, and thermoforming to obtain high-tenacity flame-retardant polyester.
According to the post-processing method provided by the invention, under the
effect of supercritical CO2,
the flame retardant can well penetrate to the surface and inside of fiber, and
the soaked fiber can be subjected to high-power drafting, so that the tenacity
of the polyester fiber is improved while the flame resistance of the polyester
fiber is improved. The post-processing method has the advantages of economical
efficiency, environmental friendliness, controllable reaction, short reaction
time and the like and has great industrial application values. The limited
oxygen index of the high-tenacity polyester obtained by the post-processing
method provided by the invention is more than 35.0%.
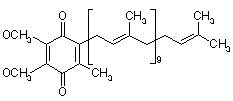
Method for preparing novel nicotianasesterpene H having antiviral activity with supercritical fluidchromatography
Abstract of CN105175240 (A)
The invention discloses a sesquiterpenoids compound which is of a novel
structure and found in tobacco. The compound is named nicotianasesterpene H, the
molecular formula of the compound is C<15>H<20>O<3>, and the structural formula
can be found in the specification. The invention further discloses the
application of the compound. Activity tests indicate that the compound has a
good inhibiting effect on rotavirus and can serve as a lead anti-rotavirus
compound for being used in research and development of anti-rotavirus medicinal
preparations.
Treatment method for coal-gasification wastewater biochemical sludge
Abstract of CN105174675 (A)
The invention provides a treatment method for coal-gasification wastewater
biochemical sludge. The method comprises the following steps: 1) transporting
the coal-gasification wastewater biochemical sludge into a heating furnace and
carrying out preheating; meanwhile, transporting liquid oxygen into a condenser
and carrying out heat exchanging and then carrying out transporting into an
oxygen buffer tank; 2) respectively allowing the preheated coal-gasification
wastewater biochemical sludge and an oxidant from the oxygen buffer tank to
enter a supercritical water
oxidation reactor, and carrying out oxidation reaction; 3) cooling the oxidized
high-temperature fluid via
a heat exchanger and then carrying out transporting to a high-pressure
gas-liquid separator; 4) discharging a separated liquid-phase product from the
bottom part of the high-pressure gas-liquid separator, and transporting a
gas-phase product to a dehydrating tower from the top part of the high-pressure
gas-liquid separator; 5) cooling the dehydrated gas-phase product through heat
exchanging of the condenser, and carrying out transporting to a CO2 purification
tower; and 6) discharging the separated liquid-phase CO2 from the bottom part of
the CO2 purification tower, and transporting the oxygen in the gas-phase product
back to the oxygen buffer tank from the top part of the CO2 purification tower,
wherein the oxygen is circularly used as the oxidant. The treatment method for
the coal-gasification wastewater biochemical sludge provided by the invention
realizes rapid and thorough degradation of organic matters in the
coal-gasification wastewater biochemical sludge; meanwhile, cyclic utilization
of the oxygen and recovery of the CO2 in the treatment process are realized, so
energy is maximumly utilized.
Powdery black food and preparation method thereof
Abstract of CN105166900 (A)
The invention discloses a powdery black food. The powdery black food is prepared
from the following raw materials in parts by weight: 20-30 parts of black rice,
20-30 parts of rye, 15-20 parts of black soybeans, 10-15 parts of black corns,
5-10 parts of black sesames, 5-8 parts of dried black fungi, 4-6 parts of kelps,
3-5 parts of black dates and 2-4 parts of figs. The powdery black food is
prepared by the following preparation steps: (1) metering and weighing according
to ratios; (2) finely grinding the metered and weighed raw materials and
sieving; and (3) after finely grinding and sieving, and carrying out superfine
crushing on the raw materials, wherein in superfine grinding, asupercritical impinging
stream crushing technology is adopted, carbon dioxide is used as main fluid,
the pressure is 15-20MPa, and the grain diameter of the crushed powdery black
food is 15-20 microns. For the powdery black food, the raw materials of the
black food are combined with the supercritical impinging
stream crushing technology to create a new food preparation technology and the
produced food has high nutritive value.
Decoloration method for yak hairs
Abstract of CN105155254 (A)
The invention relates to a decoloration method for yak hairs. The decoloration
method is characterized by comprising the following steps: starching and
finishing the yak hairs, then performing ultrasonic treatment, and performing
decoloration finishing in supercritical carbon
dioxide fluid,
wherein the whiteness of yak hair fibers obtained by decoloration is 85-95
percent, and the breaking strength is higher than or equal to 1.60cN/tex.
Supercritical carbon
dioxide ox horn button dyeing kettle and dyeing technology
Abstract of CN105155177 (A)
The invention discloses a supercritical carbon
dioxide ox horn button dyeing kettle and a dyeing technology. The dyeing kettle
comprises a kettle body. A carbon oxide fluid and
dye inlet and a carbon oxide fluid and
dye outlet are formed in the bottom of the kettle body; during the dying, the
carbon oxide fluid and
dye flow into the kettle body from the bottom to impact the button and drive the
button to dye in a non-static way, so the button can move due to air flow
impact; and dying flaws of overlapped parts between buttons can be avoided. The
cylindrical bottom of the dyeing kettle is in an inverted trapezoidal structure
to enable the buttons to have contact with carbon oxide fluid with
dying paint and finish dying. Buttons with rich colors can be acquired by the
use of the dying technology; dyed products require no cleaning and drying
operations; a water-free dying process is achieved; production cost is reduced;
and dying quality is improved and environment pollution is reduced.
Preparing method for mulberry fibers
Abstract of CN105154997 (A)
The invention discloses a preparing method for mulberry fibers. The preparing
method includes the steps of impurity removing, bundling, cage loading, chemical
preprocessing, supercritical carbon
dioxide fluid degumming
processing, bio-enzyme degumming processing, washing, dewatering, oil feeding,
carding and drying, wherein the chemical preprocessing method includes the steps
of pickling processing and alkali-oxygen processing. The mulberry fibers
obtained with the preparing method are high in degumming rate, small in damage
and high in performance, and meet the modern environment-friendly requirement.
Method for purifying sodium dehydroacetate
Abstract of CN105153088 (A)
The invention discloses a process for purifying sodium dehydroacetate by using
supercritical fluid crystallization. The process comprises the following steps:
(1) spraying neutralized impurity-containing 90 DEG C sodium dehydroacetate
solution from the top of an extraction tower by using a feedstock pump, feeding
supercritical fluid to the bottom portion of the extraction tower, and
controlling the temperature of the supercritical fluid to be 33 to 36 DEG C, the
pressure to be 9 to 11MPa and the flow to be 15 to 30m<3>/min; (2) reducing the
pressure of high-pressure supercritical fluid containing dissolved sodium
dehydroacetate to be below the critical pressure of the supercritical fluid
through a throttling valve, and then enabling the supercritical fluid to enter a
separation kettle; (3) after extraction of sodium dehydroacetate, enabling the
product to sequentially pass through dehydration and drying processes. The
process for purifying sodium dehydroacetate has the advantages that the process
is simple and the equipment structure is compact; since the crystal layer is
formed on the heat transfer surface but is not suspended in liquid during the
process, the blockage of the equipment and pipes is avoided, the production
faults are reduced, the purity of the product produced by using the process is
high, the bioactivity is good and the grain size is uniform.
Nicotianasesterpene-F prepared through supercritical fluid chromatography
and application of nicotianasesterpene-F
Abstract of CN105152880 (A)
The invention discloses a sesquiterpene compound which is prepared through
supercritical fluid chromatography and is novel in structure. The compound is
named as nicotianasesterpene-F, wherein the molecular formula of
nicotianasesterpene-F is C16H20O2, and the nicotianasesterpene-F has the
following structure (please see the structure in the specification). The
invention further discloses application of the compound. Activity tests show
that nicotianasesterpene-F has a very good restraining effect on tobacco mosaic
viruses and can be used for research and development on tobacco mosaic virus
prevention pharmaceutic preparations as the lead compound for preventing tobacco
mosaic viruses.
Pre-seasoned peanut oil convenient to cook and preparation method of pre-seasoned
peanut oil
Abstract of CN105145870 (A)
The invention discloses pre-seasoned peanut oil convenient to cook. The
pre-seasoned peanut oil consists of the following raw materials in parts by
weight: 950-1000 parts of peanuts, 15-20 parts of onion stalks, 8-10 parts of
star aniseed, 6-7 parts of Chinese prickly ash, 4-6 parts of cloves, 6-9 parts
of angelica dahurica, 6-8 parts of formosan lattuce herb, 4-5 parts of lalang
grass rhizomes, 10-12 parts of sweet-scented osmanthuses, 16-18 parts of
abelmoschus esculentus, an appropriate quantity of lactic acid bacteria, and an
appropriate amount neutral protease. According to the pre-seasoned peanut oil
disclosed by the invention, extraction is performed twice, so that the
extraction rate of fat in the peanut oil can be increased; peanut meal is
puffed, so that the inner part of the peanut meal and the inner parts of other
material compositions form porous structures, and extraction of nutrient
substances is facilitated; the lactic acid bacteria and the neutral protease
which are added can reduce the content of aflatoxin in the peanut oil, and the
content of amino acid in the fat can also be increased; a supercritical carbon
dioxide fluid extraction technology is adopted, so that not only can the active
component be effectively extracted, but also the production cost can also be
reduced; seasonings of the onion stalks, the star aniseed, the Chinese prickly
ash and the like are added, so that the process of frequently adding seasonings
in the cooking process is omitted. The pre-seasoned peanut oil has the
advantages of being convenient and simple, adequate in nutrients, unique in
fragrance and the like
ISSF
2012(10th
International Symposium on Supercritical Fluid)
Welcome
“The Versatility of Supercritical Fluid Technology”
ISSF 2012 is a global conference that brings together an international group of researchers, practitioners, and industrialists who will contribute cutting-edge presentations on emerging applications and processes. The focus of this meeting is to highlight the discovery, development, and production aspects inherent with a wide range of technologies that utilize the unique capabilities of supercritical fluid solvents. This three-day Symposium is structured with parallel sessions packed with plenary, keynote, and contributed talks as well as poster sessions. The official language of the program is English.
Click
below to see the Latest News About ISSF 2012
ISSF 2012 Preliminary Scientific Program - CLICK HERE
ISSF 2012 Brochure - CLICK HERE
Committees
Executive Committee
Jerry King, University of
Arkansas, USA (Chair);Mark
McHugh, Virginia Commonwealth University, USA (Program Chair);Feral
Temelli, University of Alberta, Canada;
Keith Hutchenson, DuPont, USA;
Ram Gupta, Auburn University, USA;
Organizing Committee
Charles Eckert, Georgia Tech,
USA;Robert
Enick, University of Pittsburgh, USA;Philip
Jessop, Queen’s University, Canada;Keith
Johnston, University of Texas, USA;Erdogan
Kiran, Virginia Tech, USA;Christopher
Kitchens, Clemson University,USA;Brian
Korgel, University of Texas, USA;Michael
Matthews, University of South Carolina, USA;Chris
Roberts, Auburn University, USA;Phillip
Savage, University of Michigan, USA;Aaron
Scurto, University of Kansas, USA;Bala
Subramaniam, University of Kansas, USA;Mark
Thies, Clemson University, USA;
International Scientific
Committee
Tadafumi Adschiri, Tohoku
University, Japan;
Bushra Al-Duri, University of Birmingham, England;Elisabeth
Badens, University Paul Cezanne Aix, France;
Gerd Brunner,
TUHH, Germany;
Susana Bottinni, PLAPIQUI, Argentina;
Owen Catchpole, Industrial Research Ltd., New Zealand;
Nimir Elbashir, Texas A&M University at Qatar;
Jacques Fages, Ecole des Mines d’Albi, France;
Neil Foster, University of New South Wales, Australia;
Motonobu Goto, Kumamoto University, Japan;
Buxing Han, Chinese Academy of Sciences, China;
Elena Ibanez, CIAL-CSIC, Madrid, Spain;
Zeljko Knez, University of Maribor, Slovenia;
Youn-Woo Lee, Seoul National University, South Korea;
Angela Meireles, UNICAMP-Campinas, Brazil;Martyn
Poliakoff, University of Nottingham, England;
Ernesto Reverchon, Universita di Salerno, Italy;
Richard Smith, Tohoku University, Japan;
Irina Smirnova, TUHH, Germany;
Charlotta Turner, Lund University, Sweden;
Lourdes Vega, MATGAS, Barcelona, Spain;Nora
Ventosa, ICMAB-CSIC, Barcelona, Spain;Ki-Pung
Yoo, Sogang University, South Korea;
Topics
Abstracts are encouraged from ALL areas of SCF including, but not limited to:
Biomass and Energy-Related Conversions
Nano-
and Particle Technology
Polymers/Materials Applications and Processes
Thermodynamics-Phase Equilibria-Fluid Properties
Separation Processes
Reactions in Critical Fluids
CO2 Remediation and Environmental Aspects
Supercritical Fluids-Ionic Liquids/Coupled Media
Natural Products, Nutraceuticals, and Food-Related Materials
Process Design and Economics
Application to Inorganic Materials
Hydrothermal Processing & Inorganic Materials
Pharmaceutical-Medical Applications
Industrial Applications of Critical Fluids
Green Chemistry/Engineering and Supercritical Fluids
NOTE -- the program will end on Wednesday, May 16, at approximately 5:15 p.m.



  |
http://www.appliedseparations.com/
http://www.aurorasfc.com/Joomla/index.php/en/
http://www.thebestreactionstation.com/
http://hcc.hanwha.co.kr/english/han/ceo_welc_idx.jsp
http://iecdivision.sites.acs.org/ http://www.suflux.com/EN/index.html
http://www.isasf.net/
http://www.jascoinc.com/
http://www.lewa.com/us/en/146/
http://www.natex.at/
http://www.parrinst.com/
http://www.phasex4scf.com/
http://www.rubotherm.de/
http://www.separex.fr/
http://www.supercriticalfluids.com/
http://www.taiatsu.co.jp/
http://www.isco.com/
http://www.waters.com/waters/home.htm?locale=zh_CN
Fractionation of thyme (Thymus vulgaris L.) by
supercritical fluid extraction and chromatography Original
Research Article
The Journal of
Supercritical Fluids,
Volume 55, Issue 3, January 2011, Pages 949-954
Mónica R. García-Risco, Gonzalo Vicente, Guillermo Reglero, Tiziana Fornari
Abstract
Supercritical fluid chromatography (SFC) was employed to fractionate thyme
(Thymus vulgaris L.) extracts, which were obtained by supercritical carbon
dioxide extraction of thyme leaves. First, different supercritical extracts were
produced at 313 K and at different pressures (15, 30 and 40 MPa). Thymol, a
monocyclic terpenoid with recognized antiseptic, analgesic and anti-inflammatory
properties, was identified and quantified in the different samples by GC–MS.
Then, the supercritical extracts were fractionated by semi-preparative SFC, and
different conditions such as pressure, temperature and amount of cosolvent
(ethanol) employed were studied. Around a two fold increase of thymol was
achieved at 15 MPa, 50 °C and 3% ethanol cosolvent, recovering 97% of the
monocyclic terpenoid extracted.
Scale-up study of
supercritical fluid extraction process for clove and sugarcane residue Original
Research Article
The Journal of
Supercritical Fluids,
Volume 56, Issue 3, April 2011, Pages 231-237
Juliana M. Prado, Glaucia H.C. Prado, M. Angela A. Meireles
Abstract
Many scale-up criteria for supercritical fluid extraction (SFE) can be found in
literature. However, the studies are often divergent and inconclusive;
therefore, more studies on this field are needed. The objective of the present
work was to study the scale-up of SFE process focusing application to Brazilian
raw materials. A laboratory scale equipment (290 mL extraction vessel) and a
pilot scale equipment (5.15 L extraction vessel) were used to study scale-up of
SFE for clove and sugarcane residue. The scale-up criterion adopted consisted in
maintaining solvent mass to feed mass ratio constant. The criterion was
successfully used for a 15-fold scale-up of overall extraction curves for both
raw materials studied; yields in pilot scale were slightly higher than in
laboratory scale. The criterion studied allows a rapid and simple scale-up
procedure, which can be very useful for the purpose of developing SFE technology
at industrial scale in developing countries where such technology is still not
available at industrial level.
Glycerol desorption from ion exchange and
adsorbent resin using supercritical fluid technology:
An optimization study Original
Research Article
The Journal of
Supercritical Fluids,
Volume 58, Issue 2, September 2011, Pages 226-232
A.E. Costa, A. Santana, M.B. Quadri, R.A.F. Machado, F. Recasens, M.A. Larrayoz
Abstract
In this study, glycerol desorption from Purolite® PD206 resin was
investigated using conventional and supercritical fluids (SCF) techniques.
Untreated biodiesel was purified by dry washing using the resin and, after
purification, the glycerol desorption was carried out using absolute ethanol
under atmospheric conditions at different mass flows (10–30 g/min) or using
ethanol-modified supercritical CO2 (1:3 molar ratio of ethanol:CO2),
under a pressure of 140 bar, within a temperature range of 106–134 °C and with
mass flow rates of 6–34 g/min. The results showed that ethanol is an efficient
solvent for this process and that the supercritical desorption is much faster
than conventional desorption process. Employing the Response Surface Methodology
(RSM) it was found that temperature has the greatest effect on the resin
regeneration time using supercritical fluids. Optimum conditions obtained were
106.1 °C and 21.9 g/min, in which the resin was regenerated in only 4.17 min.
Biological activities of Solanum paludosum
Moric. extracts obtained by maceration and supercritical fluid
extraction Original
Research Article
The Journal of
Supercritical Fluids,
Volume 58, Issue 3, October 2011, Pages 391-397
Silvia Siqueira, Vivyanne dos Santos Falcão-Silva, Maria de Fátima Agra, Claudio
Dariva, José Pinto de Siqueira-Júnior, Maria José Vieira Fonseca
Abstract
Solanum paludosum extracts obtained by maceration (SP-EtOHCRUDE and
SP-EtOHMARC) and supercritical fluid extraction () were evaluated.
Three extracts with distinct chromatographic profiles were obtained: SP-EtOHCRUDE,
with a complex phenolic composition; , containing methoxylated flavonoids; and
SP-EtOHMARC, which bears mainly polar polyphenols. All extracts were
strong antioxidants, as measured using the diphenylpicrylhydrazyl (DPPH) method,
xanthine/xanthine oxidase/luminol and iron-induced lipid peroxidation systems.
Extracts were also evaluated as modifiers of antibiotic activity in strains of
Staphylococcus aureus expressing fluoroquinolone, macrolide and tetracycline
efflux proteins. Despite their lack of antibacterial activity, all extracts were
able to potentiate antibiotics activities by as much as eight fold. Both the
maceration and supercritical fluid extraction processes were adequate to obtain
extracts with different biological activities according to their patterns of
phenolic compounds. The data suggest that S. paludosum could be a valuable
source of antioxidants and putative efflux pumps inhibitors.
Determination of sudan dyes in food samples using
supercritical fluid extraction–capillary liquid chromatography Original
Research Article
The Journal of Supercritical Fluids, Volume 55, Issue
3, January 2011, Pages 977-982
Mónica Ávila, Mohammed Zougagh, Alberto Escarpa, Ángel Ríos
Abstract
Determination of sudan dyes (Sudan I, Sudan II, Sudan III and Sudan IV) in food
samples has been developed by several and different methods. However, sample
treatment continues being the most important problem to determine these
compounds in real samples. A rapid, sensitive and selective method for the
extraction, separation and quantification of sudan dyes in commercial food
samples has been developed. The method is based on the combination of a
supercritical fluid extraction (SFE) procedure, followed by the analysis of the
extracted plug by capillary liquid chromatography (CLC) with diode array
detection (DAD). The entire procedure was optimized for the extraction of the
samples, separation and detection of the analytes. For the SFE, the effect of CO2
flow rate, extraction pressure, extraction temperature, equilibration and
extraction time and methanol modifier content were studied. Linear responses in
the range from 50 to 1000 ng mL−1 with average relative standard
deviation lower than 11.6, and detection limits ranging from 23.2 to 42.0 ng mL−1
were obtained for the different sudan dyes. The recoveries were in the range of
91–109% for dyes powder samples.
Supercritical fluid
extraction of wormwood (Artemisia absinthium L.) Original
Research Article
The Journal of
Supercritical Fluids,
Volume 56, Issue 1, February 2011, Pages 64-71
L. Martín, A.M. Mainar, A. González-Coloma, J. Burillo, J.S. Urieta
Abstract
The objective of the work was to optimize the extraction of wormwood oil by
supercritical fluid extraction (SFE) of growth-controlled plant material.
Different extraction conditions, two growth techniques and various crops were
tested and the evolution of the extracted oil composition was screened
chromatographically. A comparison with conventional techniques such as
hydrodistillation (HD) or organic solvent extraction (OSE) was also presented.
Particularly, six CO2 densities ranging from 285.0 kg/m3
to 819.5 kg/m3 were studied in the range of 9.0–18.0 MPa and 40–50
°C. A systematic study was carried out with plant material from 2005, while SFE
of 2006, 2008 and aeroponically grown crops was performed for comparative
purposes. The effect of ethanol as a modifier of the supercritical fluid
extraction was also studied. The major compounds found in the SFE extracts as
well as in the HD essential oils were Z-epoxyocimene, chrysanthenol and
chrysanthenyl acetate. A model based on mass transfer equations, the Sovová
model, was successfully applied to correlate the experimental data.
Supercritical fluid
conversion of graphene oxides Original
Research Article
The Journal of
Supercritical Fluids,
In Press, Corrected Proof,
Available online 29 September 2011
Chang Yi Kong, Wei-Li Song, Mohammed J. Meziani, Kenneth N. Tackett II, Li Cao,
Andrew J. Farr, Ankoma Anderson, Ya-Ping Sun
Abstract
In the preparation of graphene sheets for various studies and applications, the
indirect route through the reduction of graphene oxides (GOs) has been widely
pursued. Exfoliated GOs are shown to be mostly single-layer sheets in aqueous
solution, and they are also demonstrated for conversion to recover some of the
properties intrinsic to graphene. Beyond the commonly used thermal annealing and
chemical reduction methods, several environmentally friendly conversion
strategies have been explored in the literature. Reported here is a method of
annealing GOs in supercritical fluids (SCFs, including carbon dioxide and
ethanol) at relatively lower temperatures (up to only 300 °C) for their
conversion to reduced GOs (rGOs). The characteristic properties of SCFs include
low densities (thus low viscosity/high diffusivity) and diminished surface
tension, which have found successful applications in extraction and the cleaning
of fragile electronic devices, and also found to enable lower-temperature
crystallization of amorphous nanomaterials in a fluid-assisted calcination
process. In this study the same principles for lower-temperature calcination in
SCFs were applied to the conversion of GOs. The rGOs thus obtained were
characterized, with their electrical and thermal conductive properties evaluated
and correlated with the different processing conditions. The benefits and
shortcomings of the SCF processing method are discussed.
Coenzyme Q10 nanoparticles prepared
by a supercritical fluid-based method Original
Research Article
The Journal of
Supercritical Fluids,
Volume 57, Issue 1, May 2011, Pages 66-72
Xiaohui Hu, Yanni Guo, Lei Wang, Dan Hua, Yanzhen Hong, Jun Li
Abstract
A supercritical fluid-based method is proposed to produce coenzyme Q10
(CoQ10) nanoparticles. First, CoQ10/polyethylene glycol
6000 composite particles are prepared by a modified PGSS (particles from
gas-saturated solutions) process with controlling the flow rate of the
gas-saturated solution. Then, CoQ10 nanoparticles are obtained by
dissolving the composite particles into water. The effect of experimental
variables of the modified PGSS process, including pressure, temperature, flow
rate of the gas-saturated solution, and mass fraction of CoQ10, on
the CoQ10 particle size and particle size distribution was
investigated. Results show that CoQ10 slurry product with a median
diameter of 190 nm and yield of 89.8% can be prepared at an optimum condition
(operating pressure of 25 MPa, operating temperature of 80 °C, gas-saturated
solution flow rate of 1.02 mL/min, CoQ10 mass fraction of 40% and
nozzle diameter of 100 μm) via the supercritical fluid-based method.
Formulation development of Carbamazepine–Nicotinamide
co-crystals complexed with γ-cyclodextrin using supercritical
fluid process Original
Research Article
The Journal of
Supercritical Fluids,
Volume 55, Issue 3, January 2011, Pages 1070-1078
Abhijat Shikhar, Murali Mohan Bommana, Simerdeep Singh Gupta, Emilio Squillante
Abstract
The objective of the present study was to formulate and characterize co-crystals
of Carbamazepine (CBZ) and Nicotinamide (NCT) and inclusion complexes of these
co-crystals with γ-cyclodextrin (CD). Gas anti-solvent method of supercritical
fluid process (SCF) was used as the method of preparation. Formulations were
evaluated by dissolution studies, differential scanning calorimetry, hot stage
microscopy, scanning electron microscopy, 1H NMR and X-ray powder
diffraction. The dissolution studies show a 2.5-fold increase in dissolution
rate in the case of co-crystals and a 40-fold increase when co-crystals were
complexed with CD. A lower melting point (160 °C) was observed in the case of
co-crystals and the exothermic peaks were missing for pure CBZ and co-crystals
when they were complexed with CD. The absence of the melting peaks indicates
complete complexation. Electron microscopy picture shows the absence of
crystallinity in case of inclusion complexes and also the change in crystal
structure for CBZ and NCT in the case of co-crystals. X-ray powder diffraction
patterns of co-crystals and inclusion complexes were distinct from the starting
materials and the shift in peaks of 1H NMR confirmed intermolecular
hydrogen bonding and complexation
==========================================================================================================
| English Abstract of Patent |
| Approximately 5,059 results found in the Worldwide database for: |
| supercritical fluid in the title or abstract |
| Only the first 500 results are displayed |